Lignin-Mediated Silver Nanoparticle Synthesis for Photocatalytic Degradation of Reactive Yellow 4G and In Vitro Assessment of Antioxidant, Antidiabetic, and Antibacterial Activities
Abstract
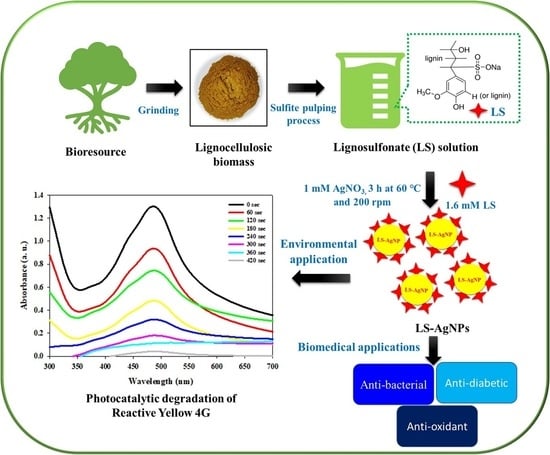
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Preparation of Ls–Ag NPs
2.3. Characterization of LS–Ag NPs
2.4. Photocatalytic Degradation of Reactive Yellow 4G by Synthesized LS–Ag NPs
2.5. In Vitro Biological Activities (Antidiabetic, Antioxidant, and Antibacterial) of Synthesized LS–Ag NPs
2.6. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of Lignosulfonate Mediated Ag NPs and Optimization of Conditions
3.2. Analytical Characterization of Synthesized LS–Ag NPs
3.2.1. XRD Analysis
3.2.2. FT-IR Analysis
3.2.3. HR-TEM Analysis
3.3. Photocatalytic Degradation of RY4G Using LS–Ag NPs and Optimization of Reaction Conditions
3.4. Recyclability of LS–Ag NPs
3.5. In Vitro Antioxidant and Antidiabetic Activity of Synthesized LS–Ag NPs
In Vitro Antibacterial Studies
3.6. Advantages of the LS–Ag NPs and Future Research Perspectives
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tang, Q.; Qian, Y.; Yang, D.; Qiu, X.; Qin, Y.; Zhou, M. Lignin-Based Nanoparticles: A Review on Their Preparations and Applications. Polymers 2020, 12, 2471. [Google Scholar] [CrossRef] [PubMed]
- Płócienniczak, P.; Rębiś, T.; Nowicki, M.; Milczarek, G. A green approach for hybrid material preparation based on carbon nanotubes/lignosulfonate decorated with silver nanostructures for electrocatalytic sensing of H2O2. J. Electroanal. Chem. 2021, 880, 114896. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Cho, S.K.; Ghodake, G.; Kadam, A.; Kumar, S.; Mulla, S.I.; Kim, D.S.; Jeon, B.H.; Chang, J.S.; et al. Phytofabrication of silver nanoparticles by Acacia nilotica leaves: Investigating their antineoplastic, free radical scavenging potential and application in H2O2 sensing. J. Taiwan Inst. Chem. Eng. 2019, 99, 239–249. [Google Scholar] [CrossRef]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Saratale, G.D.; Saratale, R.G.; Varjani, S.; Cho, S.K.; Ghodake, G.S.; Kadam, A.; Mulla, S.I.; Bharagava, R.N.; Kim, D.S.; Shin, H.S. Development of ultrasound aided chemical pretreatment methods to enrich saccharification of wheat waste biomass for polyhydroxybutyrate production and its characterization. Ind. Crops Prod. 2020, 150, 112425. [Google Scholar] [CrossRef]
- Ponnusamy, V.K.; Nguyen, D.D.; Dharmaraja, J.; Shobana, S.; Banu, J.R.; Saratale, R.G.; Chang, S.W.; Kumar, G. A review on lignin structure, pretreatments, fermentation reactions and biorefinery potential. Bioresour. Technol. 2019, 271, 462–472. [Google Scholar] [CrossRef]
- Ahvazi, B.; Cloutier, É.; Wojciechowicz, O.; Ngo, T.D. Lignin profiling: A guide for selecting appropriate lignins as precursors in biomaterials development. ACS Sustain. Chem. Eng. 2016, 4, 5090–5105. [Google Scholar] [CrossRef]
- Gao, W.J.; Fatehi, P. Lignin for polymer and nanoparticle production: Current status and challenges. Can. J. Chem. Eng. 2019, 97, 2827–2842. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Yasuda, S.; Hamaguchi, E.; Matsushita, Y.; Goto, H.; Imai, T. Ready chemical conversion of acid hydrolysis lignin into water-soluble lignosulfonate II: Hydroxymethylation and subsequent sulfonation of phenolized lignin model compounds. J. Wood Sci. 1988, 44, 116–124. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Qin, Y.; Yu, L.; Wu, R.; Yang, D.; Qiu, X.; Zhu, J.Y. Biorefinery lignosulfonates from sulfite-pretreated softwoods as dispersant for graphite. ACS Sustain. Chem. Eng. 2016, 4, 2200–2205. [Google Scholar] [CrossRef]
- Aro, T.; Fatehi, P. Production and application of Lignosulfonates and sulfonated lignin. ChemSusChem 2017, 10, 1861–1877. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Yan, J.; Yao, L.; Liu, H.; Guan, J.; Wang, H.; Liu, H. Preparation and characterization of antibacterial paper coated with sodium lignosulfonate stabilized ZnO nanoparticles. RSC Adv. 2016, 6, 9753–9759. [Google Scholar] [CrossRef]
- Yang, J.; Liu, L.; An, X.; Seta, F.T.; Li, C.; Zhang, H.; Luo, B.; Hu, Q.; Zhang, R.; Nie, S.; et al. Facile preparation of lignosulfonate induced silver nanoparticles for high efficient removal of organic contaminants in wastewater. Ind. Crops Prod. 2021, 169, 113644. [Google Scholar] [CrossRef]
- Qiao, X.G.; Wu, H.J.; Zhou, Z.; Tang, Q.Q.; Pang, X.C.; Zang, M.X.; Zhou, S.Z. Simple and facile preparation of lignosulfonate-based composite nanoparticles with tunable morphologies: From sphere to vesicle. Ind. Crops Prod. 2019, 135, 64–71. [Google Scholar] [CrossRef]
- Modrzejewska-Sikorska, A.; Konował, E.; Cich, Y.A.; Nowicki, M.; Jesionowski, T.; Milczarek, G. The effect of silver salts and lignosulfonates in the synthesis of lignosulfonate-stabilized silver nanoparticles. J. Mol. Liq. 2017, 240, 80–86. [Google Scholar] [CrossRef]
- Milczarek, G.; Rebis, T.; Fabianska, J. One-step synthesis of lignosulfonate-stabilized silver nanoparticles. Colloids Surf. B Biointerfaces 2013, 105, 335–341. [Google Scholar] [CrossRef]
- Arvinte, A.; Ignat, M.; Pinteala, M.; Ignat, L. Electrochemical Survey of Silver Nanoparticles-Lignosulfonate Formation and their Assessment in the Electrocatalytic Oxidation of P-Nitrophenol. Curr. Anal. Chem. 2017, 13, 370–378. [Google Scholar] [CrossRef]
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, J.R.; Shin, H.S. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef]
- Karade, V.C.; Patil, R.B.; Parit, S.B.; Kim, J.H.; Chougale, A.D.; Dawkar, V.V. Insights into Shape-Based Silver Nanoparticles: A Weapon to Cope with Pathogenic Attacks. ACS Sustain. Chem. Eng. 2021, 9, 12476–12507. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Ahn, S.; Shin, H.S. Grape Pomace Extracted Tannin for Green Synthesis of Silver Nanoparticles: Assessment of Their Antidiabetic, Antioxidant Potential and Antimicrobial Activity. Polymers 2021, 13, 4355. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabudhe, M.M.; Saratale, R.G.; Saratale, G.D.; Pathade, G.R. Decolorization and detoxification of sulfonated toxic diazo dye C.I. Direct Red 81 by Enterococcus faecalis YZ 66. J. Environ. Health Sci. Eng. 2014, 12, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Chandra, R.; Bhargava, R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Veisi, H.; Azizi, S.; Mohammadi, P. Green synthesis of the silver nanoparticles mediated by Thymbra spicata extract and its application as a heterogeneous and recyclable nanocatalyst for catalytic reduction of a variety of dyes in water. J. Clean. Prod. 2018, 170, 1536–1543. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Vinay, S.P.; Chandrasekhar, N. Facile Green Chemistry Synthesis of Ag Nanoparticles Using Areca Catechu Extracts for the Antimicrobial Activity and Photocatalytic Degradation of Methylene Blue Dye. Mater. Today Proc. 2019, 9, 499–505. [Google Scholar] [CrossRef]
- Hamidi, A.; Taghavizadeh Yazdi, M.E.; Amiri, M.S.; Hosseini, H.A.; Darroudi, M. Biological synthesis of silver nanoparticles in Tribulus terrestris L. extract and evaluation of their photocatalyst, antibacterial, and cytotoxicity effects. Res. Chem. Intermed. 2019, 45, 2915–2925. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Chen, J.; Amarnath, M.; Tungare, K.; Bhori, M.; Divya, M.; González-Sánchez, Z.I.; Durán-Lara, E.F.; Vaseeharan, B. Cytotoxicity, phytotoxicity, and photocatalytic assessment of biopolymer cellulose-mediated silver nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127270. [Google Scholar] [CrossRef]
- Nasab, N.K.; Sabouri, Z.; Ghazal, S.; Darroudi, M. Green-based synthesis of mixed-phase silver nanoparticles as an effective photocatalyst and investigation of their antibacterial properties. J. Mol. Struct. 2020, 1203, 127411. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Li, C.; Nabeel, F.; Khalid, M.; Iqbal, H.M.N. Catalytic potential of bio-synthesized silver nanoparticles using Convolvulus arvensis extract for the degradation of environmental pollutants. J. Photochem. Photobiol. B 2018, 181, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, R.; Joseph, S.; Mathew, B. Green synthesis of silver nanoparticles using Nervalia zeylanica leaf extract and evaluation of their antioxidant, catalytic, and antimicrobial potentials. Part. Sci. Technol. 2019, 37, 809–819. [Google Scholar] [CrossRef]
- Buthiyappan, A.; Aziz, A.R.A.; Daud, W.M.A.W. Recent advances and prospects of catalytic advanced oxidation process in treating textile effluents. Rev. Chem. Eng. 2016, 32, 1–47. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Chang, J.S.; Govindwar, S.P. Fixed-bed decolorization of Reactive Blue 172 by Proteus vulgaris NCIM-2027 immobilized on Luffa cylindrica sponge. Int. Biodeterior. Biodegrad. 2011, 65, 494–503. [Google Scholar] [CrossRef]
- Balan, K.; Qing, W.; Wang, Y.; Liu, X.; Palvannan, T.; Wang, Y.; Ma, F.; Zhang, Y. Antidiabetic activity of silver nanoparticles from green synthesis using Lonicera japonica leaf extract. RSC Adv. 2016, 6, 40162–40168. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Duygu, D.Y.; Erkaya, I.A.; Erdem, B.; Yalçin, D. Characterization of silver nanoparticle produced by Pseudopediastrum boryanum (Turpin) E. Hegewald and its antimicrobial effects on some pathogens. Int. J. Environ. Sci. Technol. 2019, 16, 7093–7102. [Google Scholar] [CrossRef]
- Chen, S.; Wang, G.; Pang, T.; Sui, W.; Chen, Z.; Si, C. Green assembly of high-density and small-sized silver nanoparticles on lignosulfonate-phenolic resin spheres: Focusing on multifunction of lignosulfonate. Int. J. Biol. Macromol. 2021, 166, 893–901. [Google Scholar] [CrossRef]
- Ahmadpoor, F.; Nasrollahzadeh, M.; Mohammad, M. Self-assembled lignosulfonate-inorganic hybrid nanoflowers and their application in catalytic reduction of methylene blue and 4-nitrophenol. Separat. Purif. Technol. 2021, 272, 118864. [Google Scholar] [CrossRef]
- Shankar, S.S.; Ahmad, A.; Sastry, M. Geranium leaf assistedbiosynthesis of silver nanoparticles. Biotechnol. Prog. 2003, 19, 1627–1631. [Google Scholar] [CrossRef]
- Daizy, P Mangifera Indica leaf-assisted biosynthesis of well-dispersed silver nanoparticles. Spectrochim. Acta Part A 2011, 78, 327–331. [CrossRef] [PubMed]
- Vanaja, M.; Annadurai, G. Coleus aromaticus leaf extract mediated synthesis of silver nanoparticles and its bactericidal activity. Appl. Nanosci. 2013, 3, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Abdulkhani, A.; Amiri, E.; Sharifzadeh, A.; Hedjazi, S.; Alizadeh, P. Concurrent production of sodium lignosulfonate and ethanol from bagasse spent liquor. J. Environ. Manag. 2019, 231, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Z.; Guo, X.; He, Q.; Hao, C.; Ge, C. Ultrasonic-assisted synthesis of sodium lignosulfonate-grafted poly(acrylic acid-co-poly(vinyl pyrrolidone)) hydrogel for drug delivery. RSC Adv. 2016, 6, 35550–35558. [Google Scholar] [CrossRef]
- Orlowski, P.; Zmigrodzka, M.; Tomaszewska, E.; Soliwoda, K.; Czupryn, M.; Antos-Bielska, M.; Szemraj, J.; Celichowski, G.; Grobelny, J.; Krzyzowska, M. Tannic acid-modified silver nanoparticles for wound healing: The importance of size. Int. J. Nanomed. 2018, 13, 991–1007. [Google Scholar] [CrossRef] [Green Version]
- Chahardoli, A.; Qalekhani, F.; Shokoohinia, Y.; Fattahi, A. Biological and catalytic activities of green synthesized silverna-noparticles from the leaf infusion of Dracocephalum kotschyi. Boiss. Glob. Chall. 2021, 5, 2000018. [Google Scholar] [CrossRef]
- Gao, F.; Yu, S.; Tao, Q.; Tan, W.; Duan, L.; Li, Z.; Cui, H. Lignosulfonate improves photostability and bioactivity of abscisic acid under ultraviolet radiation. J. Agric. Food Chem. 2018, 66, 6585–6593. [Google Scholar] [CrossRef]
- Subash, B.; Krishnakumar, B.; Swaminathan, M.; Shanthi, M. ZnS–Ag–ZnO as an excellent UV-light-active photocatalyst for the degradation of AV 7, AB 1, RR 120, and RY 84 dyes: Synthesis, characterization, and catalytic applications. Ind. Eng. Chem. Res. 2014, 53, 12953–12963. [Google Scholar] [CrossRef]
- Sasikala, R.; Karthikeyan, K.; Easwaramoorthy, D.; Bilal, I.M.; Rani, S.K. Photocatalytic degradation of trypan blue and methyl orange azo dyes by cerium loaded CuO nanoparticles. Environ. Nanotechnol. Monit. Manag. 2016, 6, 45–53. [Google Scholar] [CrossRef]
- Sapkal, R.T.; Shinde, S.S.; Mahadik, M.A.; Mohite, V.S.; Waghmode, T.R.; Govindwar, S.P.; Bhosale, C.H. Photoelectrocatalytic decolorization and degradation of textile effluent using ZnO thin films. J. Photochem. Photobiol. B 2012, 114, 102–107. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Dutta, B.K. Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor catalyst. J. Hazard. Mater. 2004, 112, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Tang, J.; Liu, X.; Ren, X.; Zhen, M.; Wang, L. Green Biosynthesis of Silver Nanoparticles Using Eriobotrya japonica (Thunb.) Leaf Extract for Reductive Catalysis. Materials. 2019, 12, 189. [Google Scholar] [CrossRef] [Green Version]
- Saratale, R.G.; Ghodake, G.S.; Shinde, S.K.; Cho, S.K.; Saratale, G.D.; Pugazhendhi, A.; Bharagava, R.N. Photocatalytic activity of CuO/Cu(OH)2 nanostructures in the degradation of Reactive Green 19A and textile effluent, phytotoxicity studies and their biogenic properties (antibacterial and anticancer). J. Environ. Manag. 2018, 223, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Saratale, G.D.; Saratale, R.G.; Cho, S.-K.; Ghodake, G.; Bharagava, R.N.; Park, Y.; Mulla, S.I.; Kim, D.-S.; Kadam, A.; Nair, S.; et al. Investigation of photocatalytic degradation of reactive textile dyes by Portulaca oleracea-functionalized silver nanocomposites and exploration of their antibacterial and antidiabetic potentials. J. Alloys Compd. 2020, 833, 155083. [Google Scholar] [CrossRef]
- Riyaphan, J.; Pham, D.-C.; Leong, M.K.; Weng, C.-F. In Silico Approaches to Identify Polyphenol Compounds as α-Glucosidase and α-Amylase Inhibitors against Type-II Diabetes. Biomolecules 2021, 11, 1877. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, L.; Dong, Y.; Fang, Z.; Nisar, T.; Zhao, T.; Wang, Z.C.; Guo, Y. Chemical compositions and α-glucosiase inhibitory effects of anthocyanidins from blueberry, blackcurrant and blue honeysuckle fruits. Food Chem. 2019, 299, 125102. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, K.; Sureshkumar, P. Green synthesis of silver nanoparticle using Tephrosia tinctoria and its anti-diabetic activity. Mater. Lett. 2015, 138, 251–254. [Google Scholar] [CrossRef]
- Govindappa, M.; Arthikala, M.K.; Rai, V.R.; Ramachandra, Y.L. Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllum tomentosum leaves extract. Results Phys. 2018, 9, 400–408. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, S.; Srivastava, B.; Bhadouria, R.; Singh, R. Green synthesis of silver nanoparticles using leaf extract of Holoptelea integrifolia and preliminary investigation of its antioxidant, anti-inflammatory, antidiabetic and antibacterial activities. J. Environ. Chem. Eng. 2019, 7, 103094. [Google Scholar] [CrossRef]
- Dauthal, P.; Mukhopadhyay, M. Noble Metal Nanoparticles: Plant-Mediated Synthesis, Mechanistic Aspects of Synthesis, and Applications. Ind. Eng. Chem. Res. 2016, 55, 9557–9577. [Google Scholar] [CrossRef]
- Kim, S.; Fernandes, M.M.; Matamá, T.; Loureiro, A.; Gomes, A.C.; Cavaco-Paulo, A. Chitosan-lignosulfonates sono-chemically prepared nanoparticles: Characterisation and potential applications. Colloids Surf. B Biointerfaces. 2013, 103, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Marulasiddeshwara, M.B.; Dakshayani, S.S.; Sharath Kumar, M.N.; Chethana, R.; Raghavendra Kumar, P.; Devaraja, S. Facile-one pot-green synthesis, antibacterial, antifungal, antioxidant and antiplatelet activities of lignin capped silver nanoparticles: A promising therapeutic agent. Mater Sci. Eng. C 2017, 81, 182–190. [Google Scholar] [CrossRef] [PubMed]










| Parameter | Lignosulfonate |
|---|---|
| Treatment conditions | Metal sulfite + sulfur dioxide (Ca2+, Mg2+ or Na+) (pH = 2–12, T = 120–180 °C, for 1–5 h) |
| Solubility | Water |
| Ash content (mass %) | 4.0–9.3 |
| Sulfur (%) | 3.5–8.0 |
| Carbohydrates (mass %) | ND |
| Molecular weight (Da) | 1000–50,000 |
| Polydispersity Index (PDI) | 4.2–8.0 |
| Zone of Inhibition (mm) | ||||
|---|---|---|---|---|
| Bacteria Strain | LS–Ag NPs (20 μg/mL) | Ampicillin (20 μg/mL) | Sodium Lignosulfonate (20 μg/mL) | Antimicrobial Index (%) |
| Escherichia coli | 15.8 ± 0.38 | 16.8 ± 0.35 | 4.45 ± 0.45 | 94.0 ± 2.45 |
| Staphylococcus aureus | 12.2 ± 0.54 | 14.2 ± 0.41 | 3.25 ± 0.23 | 85.9 ± 2.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saratale, R.G.; Cho, S.-K.; Saratale, G.D.; Kadam, A.A.; Ghodake, G.S.; Magotra, V.K.; Kumar, M.; Bharagava, R.N.; Varjani, S.; Palem, R.R.; et al. Lignin-Mediated Silver Nanoparticle Synthesis for Photocatalytic Degradation of Reactive Yellow 4G and In Vitro Assessment of Antioxidant, Antidiabetic, and Antibacterial Activities. Polymers 2022, 14, 648. https://doi.org/10.3390/polym14030648
Saratale RG, Cho S-K, Saratale GD, Kadam AA, Ghodake GS, Magotra VK, Kumar M, Bharagava RN, Varjani S, Palem RR, et al. Lignin-Mediated Silver Nanoparticle Synthesis for Photocatalytic Degradation of Reactive Yellow 4G and In Vitro Assessment of Antioxidant, Antidiabetic, and Antibacterial Activities. Polymers. 2022; 14(3):648. https://doi.org/10.3390/polym14030648
Chicago/Turabian StyleSaratale, Rijuta Ganesh, Si-Kyung Cho, Ganesh Dattatraya Saratale, Avinash Ashok Kadam, Gajanan Sampatrao Ghodake, Verjesh Kumar Magotra, Manu Kumar, Ram Naresh Bharagava, Sunita Varjani, Ramasubba Reddy Palem, and et al. 2022. "Lignin-Mediated Silver Nanoparticle Synthesis for Photocatalytic Degradation of Reactive Yellow 4G and In Vitro Assessment of Antioxidant, Antidiabetic, and Antibacterial Activities" Polymers 14, no. 3: 648. https://doi.org/10.3390/polym14030648
APA StyleSaratale, R. G., Cho, S. -K., Saratale, G. D., Kadam, A. A., Ghodake, G. S., Magotra, V. K., Kumar, M., Bharagava, R. N., Varjani, S., Palem, R. R., Mulla, S. I., Kim, D. -S., & Shin, H. -S. (2022). Lignin-Mediated Silver Nanoparticle Synthesis for Photocatalytic Degradation of Reactive Yellow 4G and In Vitro Assessment of Antioxidant, Antidiabetic, and Antibacterial Activities. Polymers, 14(3), 648. https://doi.org/10.3390/polym14030648