Phyto-Assisted Assembly of Metal Nanoparticles in Chitosan Matrix Using S. argel Leaf Extract and Its Application for Catalytic Oxidation of Benzyl Alcohol
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.2.1. Preparation of S. argel Leaf Extract
2.2.2. Cross-Linked Chitosan Preparation
2.2.3. Preparation of Chitosan-GLA/AuNPs Hybrid Nanocomposite
2.2.4. Characterization Methods
2.3. Catalytic Evaluation of Chitosan-GLA/AuNPs Hybrid Nanocomposite
3. Result and Discussion
3.1. FT-IR
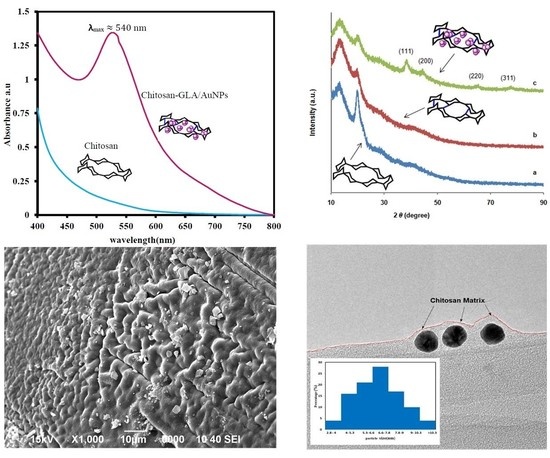
3.2. UV–vis
3.3. Elemental Analysis
3.4. XRD
3.5. FESEM
3.6. TGA
3.7. TEM
3.8. Specific Surface Area (BET)
3.9. Catalytic Activity Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, W.; Shin, H.; Choi, B.; Rhim, W.-K.; Na, K.; Han, D.K. Advanced hybrid nanomaterials for biomedical applications. Prog. Mater. Sci. 2020, 114, 100686. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, X.; He, Z. Organic–inorganic nanoflowers: From design strategy to biomedical applications. Nanoscale 2019, 11, 17179–17194. [Google Scholar] [CrossRef]
- Yu, M.-R.; Suyambrakasam, G.; Wu, R.-J.; Chavali, M. Preparation of organic–inorganic (SWCNT/TWEEN–TEOS) nano hybrids and their NO gas sensing properties. Sens. Actuators B Chem. 2012, 161, 938–947. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Zhang, M.; Zeng, B.; Zhao, F. Molecularly imprinted photoelectrochemical sensor for aflatoxin B1 detection based on organic/inorganic hybrid nanorod arrays. Sens. Actuators B Chem. 2021, 339, 129900. [Google Scholar] [CrossRef]
- Huang, G.; Zhou, H.; Wang, C.; Kashi, C.; Ye, X.; Li, W.; Wang, G.-E.; Xu, G. A new 1D inorganic–organic hybrid perovskite-like semiconductor with high stability and humidity response. Inorg. Chem. Commun. 2021, 128, 108581. [Google Scholar] [CrossRef]
- Kanaujia, P.K.; Dehury, K.M.; Adnan, M.; Prakash, G.V. Alternative fabrication methodologies for two-dimensional self-assembled Inorganic-Organic hybrid semiconductors. Opt. Mater. 2020, 110, 110511. [Google Scholar] [CrossRef]
- Salvo, A.M.P.; Giacalone, F.; Gruttadauria, M. Advances in Organic and Organic-Inorganic Hybrid Polymeric Supports for Catalytic Applications. Molecules 2016, 21, 1288. [Google Scholar] [CrossRef] [Green Version]
- Berijani, K.; Morsali, A. The role of metal–organic porous frameworks in dual catalysis. Inorg. Chem. Front. 2021, 8, 3618–3658. [Google Scholar] [CrossRef]
- Varadwaj, G.B.B.; Parida, K.; Nyamori, V.O. Transforming inorganic layered montmorillonite into inorganic–organic hybrid materials for various applications: A brief overview. Inorg. Chem. Front. 2016, 3, 1100–1111. [Google Scholar] [CrossRef]
- Budroni, G.; Corma, A. Gold–Organic–Inorganic High-Surface-Area Materials as Precursors of Highly Active Catalysts. Angew. Chem. Int. Ed. 2006, 45, 3328–3331. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Park, H.-H.; Golledge, S.; Johnson, D.C. A study on the incorporation of ZnO nanoparticles into MEH-PPV based organic–inorganic hybrid solar cells. Ceram. Int. 2012, 38, S525–S528. [Google Scholar] [CrossRef]
- Gómez-Romero, P.; Ayyad, O.; Suárez-Guevara, J.; Muñoz-Rojas, D. Hybrid organic–inorganic materials: From child’s play to energy applications. J. Solid State Electrochem. 2010, 14, 1939–1945. [Google Scholar] [CrossRef]
- Fu, H.; Jiang, C.; Luo, C.; Lin, H.; Peng, H. A Quasi-Two-Dimensional Copper Based Organic-Inorganic Hybrid Perovskite with Reversible Thermochromism and Ferromagnetism. Eur. J. Inorg. Chem. 2021, 2021, 4984–4989. [Google Scholar] [CrossRef]
- Cai, P.; Wang, S.; Xu, T.; Tang, Y.; Yuan, X.; Wan, M.; Ai, Q.; Si, J.; Yao, X.; Cao, Y.; et al. Mn4+ doped zero-dimensional organic-inorganic hybrid material with narrow-red emission. J. Lumin. 2020, 228, 117661. [Google Scholar] [CrossRef]
- Wang, G.-E.; Sun, C.; Wang, M.-S.; Guo, G.-C. Semiconducting crystalline inorganic–organic hybrid metal halide nanochains. Nanoscale 2020, 12, 4771–4789. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Chi, Q.; Dong, Y.; Liu, L.; Zhang, Y.; Chang, L.; Pan, Y.; He, A.; Li, J.; Wang, X. Effects of annealing on the magnetic properties of Fe-based amorphous powder cores with inorganic-organic hybrid insulating layer. J. Magn. Magn. Mater. 2020, 494, 165827. [Google Scholar] [CrossRef]
- Zhao, H.; Fu, H.; Hu, Z.; Fu, Q.; Tao, H.; Weng, J.; Xiong, L.; Cheng, Z. Magnetic hybrid organic–inorganic perovskite (CH3NH3)2XCl4 (X = Mn, Cu, Co) crystals. CrystEngComm 2021, 23, 5208–5213. [Google Scholar] [CrossRef]
- Liu, D.-D.; Chen, Y.-G. Coordination polymers of lanthanide elements and metatungstate: Syntheses, structure and magnetic property. Inorg. Chim. Acta 2013, 401, 70–75. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, X.; Luo, M. A novel inorganic–organic hybrid compound constructed from copper(II)-monosubstituted polyoxometalates and poly(amidoamine). J. Solid State Electrochem. 2008, 13, 1585–1589. [Google Scholar] [CrossRef]
- Vasylyev, M.V.; Gatard, S.; Bar-Nahum, I.; Konstantinovski, L.; Wachtel, E.J.; Neumann, R. Synthesis and Characterization of Polyoxometalate–Polyamino Dendritic Hybrid Compounds. J. Clust. Sci. 2006, 17, 235–243. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Z.; Cao, M.; Cao, R. Stable gold nanoparticle encapsulated in silica-dendrimers organic–inorganic hybrid composite as recyclable catalyst for oxidation of alcohol. Microporous Mesoporous Mater. 2010, 136, 42–49. [Google Scholar] [CrossRef]
- Naka, K.; Chujo, Y. Effect of anionic dendrimers on the crystallization of calcium carbonate in aqueous solution. Comptes Rendus. Chim. 2003, 6, 1193–1200. [Google Scholar] [CrossRef]
- Gil, M.; Kim, H.; Bae, J.; Cha, S.-H.; Lee, K.J. Preparation of metal-ion containing polymers: Synthesis and characterization of methacryliccopolymers containing copper ion. Polymer 2015, 77, 297–304. [Google Scholar] [CrossRef]
- Zhang, R.-C.; Sun, D.; Zhang, R.; Lin, W.-F.; Macias-Montero, M.; Patel, J.; Askari, S.; McDonald, C.; Mariotti, D.; Maguire, P. Gold nanoparticle-polymer nanocomposites synthesized by room temperature atmospheric pressure plasma and their potential for fuel cell electrocatalytic application. Sci. Rep. 2017, 7, 46682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolstov, A.L. Preparation, Structure, and Properties of Hybrid Polymer Composites Containing Silver Clusters and Nanoparticles. Theor. Exp. Chem. 2015, 51, 74–95. [Google Scholar] [CrossRef]
- Sharon, M.; Nandgavkar, I.; Sharon, M. Platinum nanocomposites and its applications: A review. Adv. Mater. Res. 2017, 2, 129–153. [Google Scholar]
- Negm, N.A.; Hefni, H.; Abd-Elaal, A.A.; Badr, E.A.; Kana, M.T.A. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.L.; Kumar, S.; Das, A.; Randhawa, J.S.; Chamundeeswari, M. Chitin and chitosan-based support materials for enzyme immobilization and biotechnological applications. Environ. Chem. Lett. 2019, 18, 315–323. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Fan, L.; Liu, H.; Hu, Y. Chitosan- metal complexes as antimicrobial agent: Synthesis, characterization and Structure-activity study. Polym. Bull. 2005, 55, 105–113. [Google Scholar] [CrossRef]
- Di Carlo, G.; Curulli, A.; Toro, R.G.; Bianchini, C.; De Caro, T.; Padeletti, G.; Zane, D.; Ingo, G.M. Green Synthesis of Gold–Chitosan Nanocomposites for Caffeic Acid Sensing. Langmuir 2012, 28, 5471–5479. [Google Scholar] [CrossRef]
- Ngah, W.W.; Fatinathan, S. Chitosan flakes and chitosan–GLA beads for adsorption of p-nitrophenol in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2006, 277, 214–222. [Google Scholar] [CrossRef]
- Geçer, A.; Yıldız, N.; Çalımlı, A.; Turan, B. Trimethyl chitosan nanoparticles enhances dissolution of the poorly water soluble drug Candesartan-Cilexetil. Macromol. Res. 2010, 18, 986–991. [Google Scholar] [CrossRef]
- Xu, X.; Liu, P.; Li, S.-H.; Zhang, P.; Wang, X.-Y. Chitosan-supported imine palladacycle complex and its catalytic performance for heck reaction. React. Kinet. Catal. Lett. 2006, 88, 217–223. [Google Scholar] [CrossRef]
- Francis, A.O.; Zaini, M.A.A.; Muhammad, I.M.; Abdulsalam, S.; El-Nafaty, U.A. Physicochemical modification of chitosan adsorbent: A perspective. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Mourya, V.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Cárdenas-Triviño, G.; Cruzat-Contreras, C. Study of Aggregation of Gold Nanoparticles in Chitosan. J. Clust. Sci. 2018, 29, 1081–1088. [Google Scholar] [CrossRef] [Green Version]
- Keshipour, S.; Mirmasoudi, S.S. Cross-linked chitosan aerogel modified with Au: Synthesis, characterization and catalytic application. Carbohydr. Polym. 2018, 196, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Zahedifar, M.; Es-Haghi, A.; Zhiani, R.; Sadeghzadeh, S.M. Synthesis of benzimidazolones by immobilized gold nanoparticles on chitosan extracted from shrimp shells supported on fibrous phosphosilicate. RSC Adv. 2019, 9, 6494–6501. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.A.; Bakhsh, E.M.; Asiri, A.M.; Khan, S.B. Synthesis of zero-valent Au nanoparticles on chitosan coated NiAl layered double hydroxide microspheres for the discoloration of dyes in aqueous medium. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 250, 119370. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.B.; Rufato, K.B.; de Oliveira, A.C.; Souza, P.R.; da Silva, E.P.; Muniz, E.C.; Vilsinski, B.H.; Martins, A.F. Composite materials based on chitosan/gold nanoparticles: From synthesis to biomedical applications. Int. J. Biol. Macromol. 2020, 161, 977–998. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2020, 19, 355–374. [Google Scholar] [CrossRef]
- Nadeem, M.; Abbasi, B.H.; Younas, M.; Ahmad, W.; Khan, T. A review of the green syntheses and anti-microbial applications of gold nanoparticles. Green Chem. Lett. Rev. 2017, 10, 216–227. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, A.D.; Gopal, K. Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf. A Physicochem. Eng. Asp. 2010, 369, 27–33. [Google Scholar] [CrossRef]
- Annamalai, A.; Christina, V.; Sudha, D.; Kalpana, M.; Lakshmi, P. Green synthesis, characterization and antimicrobial activity of Au NPs using Euphorbia hirta L. leaf extract. Colloids Surf. B Biointerfaces 2013, 108, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Mukundan, D.; Mohankumar, R.; Vasanthakumari, R. Comparative study of synthesized silver and gold nanoparticles using leaves extract of Bauhinia tomentosa Linn and their anticancer efficacy. Bull. Mater. Sci. 2017, 40, 335–344. [Google Scholar] [CrossRef]
- Hamed, A.I. New steroids from Solenostemma argel leaves. Fitoterapia 2001, 72, 747–755. [Google Scholar] [CrossRef]
- El-Shiekh, R.; Al-Mahdy, D.; Hifnawy, M.; Abdel-Sattar, E. In-vitro screening of selected traditional medicinal plants for their anti-obesity and anti-oxidant activities. S. Afr. J. Bot. 2019, 123, 43–50. [Google Scholar] [CrossRef]
- El-Zayat, M.M.; Eraqi, M.M.; Alfaiz, F.A.; Elshaer, M.M. Antibacterial and antioxidant potential of some Egyptian medicinal plants used in traditional medicine. J. King Saud Univ.-Sci. 2021, 33, 101466. [Google Scholar] [CrossRef]
- Plaza, A.; Perrone, A.; Balestrieri, C.; Balestrieri, M.L.; Bifulco, G.; Carbone, V.; Hamed, A.; Pizza, C.; Piacente, S. New antiproliferative 14,15-secopregnane glycosides from Solenostemma argel. Tetrahedron 2005, 61, 7470–7480. [Google Scholar] [CrossRef]
- Adel, R.; Saty, A.; Yousof, S.; Alhuseen, O.; Mohammed, H.; Alamin, A. Phytochemical Screening of Solenostemma argel & Extraction & Separation of Its Flavonoid. Ph.D. Thesis, Sudan University of Science and Technology, Khartoum State, Sudan, 2016. [Google Scholar]
- Pinto, R.V.; Gomes, P.S.; Fernandes, M.H.; Costa, M.E.; Almeida, M. Glutaraldehyde-crosslinking chitosan scaffolds reinforced with calcium phosphate spray-dried granules for bone tissue applications. Mater. Sci. Eng. C 2019, 109, 110557. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Shan, C.-L.; Zhou, Q.; Fang, Y.; Wang, Y.-L.; Xu, F.; Han, L.-R.; Ibrahim, M.; Guo, L.-B.; Xie, G.-L.; et al. Synthesis, Characterization, and Antibacterial Activity of Cross-Linked Chitosan-Glutaraldehyde. Mar. Drugs 2013, 11, 1534–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Pu, S.; Li, J.; Cai, J.; Zhou, B.; Ren, G.; Ma, Q.; Zhong, L. Size controllable one step synthesis of gold nanoparticles using carboxymethyl chitosan. Int. J. Biol. Macromol. 2018, 122, 770–783. [Google Scholar] [CrossRef]
- Da’Na, E.; Sayari, A. Optimization of copper removal efficiency by adsorption on amine-modified SBA-15: Experimental design methodology. Chem. Eng. J. 2011, 167, 91–98. [Google Scholar] [CrossRef]
- Huang, J.; Li, Q.; Sun, D.; Lu, Y.; Su, Y.; Yang, X.; Wang, H.; Wang, Y.; Shao, W.; He, N.; et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 2007, 18, 105104. [Google Scholar] [CrossRef]
- Sugunan, A.; Thanachayanont, C.; Dutta, J.; Hilborn, J. Heavy-metal ion sensors using chitosan-capped gold nanoparticles. Sci. Technol. Adv. Mater. 2005, 6, 335–340. [Google Scholar] [CrossRef]
- Luo, W.; Bai, Z.; Zhu, Y. Fast removal of Co(ii) from aqueous solution using porous carboxymethyl chitosan beads and its adsorption mechanism. RSC Adv. 2018, 8, 13370–13387. [Google Scholar] [CrossRef] [Green Version]










| Entry | Samples | Elements | ||||
|---|---|---|---|---|---|---|
| C% | %H | %N | Au | |||
| % atom | mmol g−1 | |||||
| 1 | Chitosan | 40.91 | 7.54 | 7.80 | - | - |
| 2 | Chitosan-GLA | 43.58 | 7.81 | 6.95 | - | - |
| 3 | Chitosan-GLA/Au3+ | 51.59 | 8.81 | 5.52 | 2.033 | 0.103 |
| 4 | Chitosan-GLA/AuNPs | 51.63 | 8.83 | 5.50 | 2.024 | 0.102 |
| Entry | Catalyst | BET (m2g−1) | Crystallinity (%) | Conversion % a |
|---|---|---|---|---|
| 1 | chitosan-GLA/AuNPs beads | 4.90 | 64.21 | 97.5 |
| 2 | No catalyst | - | - | 10.0 |
| 3 | S. argel leaf extract | - | - | 12.8 |
| 4 | chitosan flakes | 3.25 | 62.03 | 4.3 |
| 5 | chitosan-GLA beads | 4.73 | 63.18 | 14.9 |
| 6 | chitosan-GLA/Au3+ beads | - | - | 42.3 |
| 7 | chitosan-GLA/AuNPs beads | 95.1 b | ||
| 8 | chitosan-GLA/AuNPs beads | 91.3 c | ||
| 9 | chitosan-GLA/AuNPs beads | 70.0 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, A.; Da’na, E. Phyto-Assisted Assembly of Metal Nanoparticles in Chitosan Matrix Using S. argel Leaf Extract and Its Application for Catalytic Oxidation of Benzyl Alcohol. Polymers 2022, 14, 766. https://doi.org/10.3390/polym14040766
Taha A, Da’na E. Phyto-Assisted Assembly of Metal Nanoparticles in Chitosan Matrix Using S. argel Leaf Extract and Its Application for Catalytic Oxidation of Benzyl Alcohol. Polymers. 2022; 14(4):766. https://doi.org/10.3390/polym14040766
Chicago/Turabian StyleTaha, Amel, and Enshirah Da’na. 2022. "Phyto-Assisted Assembly of Metal Nanoparticles in Chitosan Matrix Using S. argel Leaf Extract and Its Application for Catalytic Oxidation of Benzyl Alcohol" Polymers 14, no. 4: 766. https://doi.org/10.3390/polym14040766
APA StyleTaha, A., & Da’na, E. (2022). Phyto-Assisted Assembly of Metal Nanoparticles in Chitosan Matrix Using S. argel Leaf Extract and Its Application for Catalytic Oxidation of Benzyl Alcohol. Polymers, 14(4), 766. https://doi.org/10.3390/polym14040766