Classification and Characterization of Tire-Road Wear Particles in Road Dust by Density
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Matrix Solution Preparation for Density Separation
2.3. Preparation of Road Dust Sample
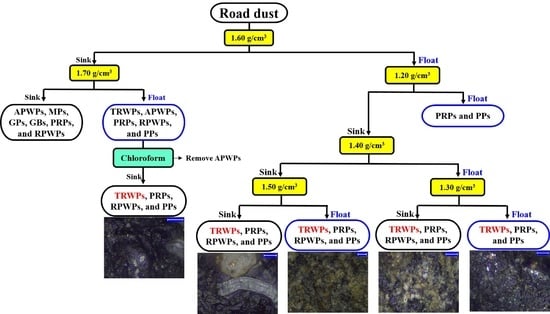
2.4. Density Separation Method
2.5. Characterization of TRWPs
3. Results and Discussion
3.1. Composition and Density Properties of the Road Dust
3.2. Differentiation of TRWPs and APWPs
3.3. Density Separation of the Road Dust Samples
3.4. Characterization of TRWPs Classified by the Density Separation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kovochich, M.; Parker, J.A.; Oh, S.C.; Lee, J.P.; Wagner, S.; Reemtsma, T.; Unice, K.M. Characterization of individual tire and road wear particles in environmental road dust, tunnel dust, and sediment. Environ. Sci. Technol. Lett. 2021, 8, 1057–1064. [Google Scholar] [CrossRef]
- Klockner, P.; Reemtsma, T.; Eisentraut, P.; Braun, U.; Ruhl, A.S.; Wagner, S. Tire and road wear particles in road environment—Quantification and assessment of particle dynamics by Zn determination after density separation. Chemosphere 2019, 222, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Kreider, M.L.; Panko, J.M.; McAtee, B.L.; Sweet, L.I.; Finley, B.L. Physical and chemical characterization of tire-related particles: Comparison of particles generated using different methodologies. Sci. Total Environ. 2010, 408, 652–659. [Google Scholar] [CrossRef]
- Jung, U.Y.; Choi, S.S. A variety of particles including tire wear particles produced on the road. Elast. Compos. 2021, 56, 85–91. [Google Scholar]
- Sakai, H. Friction and wear of tire tread rubber. Tire Sci. Technol. 1996, 24, 252–275. [Google Scholar] [CrossRef]
- Kole, P.J.; Löhr, A.J.; Van Belleghem, F.G.A.J.; Ragas, A.M.J. Wear and tear of tyres: A stealthy source of microplastics in the environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef]
- Järlskog, I.; Strömvall, A.-M.; Magnusson, K.; Gustafsson, M.; Polukarova, M.; Galfi, H.; Aronsson, M.; Andersson-Sköld, Y. Occurrence of tire and bitumen wear microplastics on urban streets and in sweepsand and washwater. Sci. Total Environ. 2020, 729, 138950. [Google Scholar] [CrossRef]
- Järlskog, I.; Strömvall, A.-M.; Magnusson, K.; Galfi, H.; Björklund, K.; Polukarova, M.; Garção, R.; Markiewicz, A.; Aronsson, M.; Gustafsson, M.; et al. Traffic-related microplastic particles, metals, and organic pollutants in an urban area under reconstruction. Sci. Total Environ. 2021, 774, 145503. [Google Scholar] [CrossRef]
- Degaffe, F.S.; Turner, A. Leaching of zinc from tire wear particles under simulated estuarine conditions. Chemosphere 2011, 85, 738–743. [Google Scholar] [CrossRef]
- Unice, K.M.; Weeber, M.P.; Abramson, M.M.; Reid, R.C.D.; Gils, J.A.G.V.; Markus, A.A.; Vethaak, A.D.; Panko, J.M. Characterizing export of land-based microplastics to the estuary—Part I: Application of integrated geospatial microplastic transport models to assess tire and road wear particles in the Seine watershed. Sci. Total Environ. 2019, 646, 1639–1649. [Google Scholar] [CrossRef]
- Lan, H.; Martin, C.D.; Hu, B. Effect of heterogeneity of brittle rock on micromechanical extensile behavior during compression loading. J. Geophys. Res. Solid Earth 2010, 115, B01202. [Google Scholar] [CrossRef]
- Sarpün, İ.H.; Kılıçkaya, M.S.; Tuncel, S. Mean grain size determination in marbles by ultrasonic velocity techniques. NDT Int. 2005, 38, 21–25. [Google Scholar] [CrossRef]
- Januszewicz, K.; Kazimierski, P.; Suchocki, T.; Kardas, D.; Lewandowski, W.; Klugmann-Radziemska, E.; Luczak, J. Waste rubber pyrolysis: Product yields and limonene concentration. Materials 2020, 13, 4435. [Google Scholar] [CrossRef] [PubMed]
- Danon, B.; van der Gryp, P.; Schwarz, C.E.; Görgens, J.F. A review of dipentene (DL-limonene) production from waste tire pyrolysis. J. Anal. Appl. Pyrolysis 2015, 112, 1–13. [Google Scholar] [CrossRef]
- Choi, S.-S.; Ko, J.-E. Influence of thermal aging on pyrolysis pattern of carbon black-filled NR composite. Macromol. Res. 2007, 15, 482–485. [Google Scholar] [CrossRef]
- Pakdel, H.; Pantea, D.M.; Roy, C. Production of dl-limonene by vacuum pyrolysis of used tires. J. Anal. Appl. Pyrolysis 2001, 57, 91–107. [Google Scholar] [CrossRef]
- Chae, E.; Jung, U.; Choi, S.-S. Quantification of tire tread wear particles in microparticles produced on the road using oleamide as a novel marker. Environ. Pollut. 2021, 288, 117811. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-S.; Yang, S.R.; Chae, E.; Son, C.E. Influence of carbon black contents and rubber compositions on formation of wear debris of rubber vulcanizates. Elast. Compos. 2020, 55, 108–113. [Google Scholar]
- Miyazaki, T. Rubber Composition. European Patent EP3459996, 21 April 2017. [Google Scholar]
- Ghoreishy, M.H.R.; Alimardani, M.; Mehrabian, R.Z.; Gangali, S.T. Modeling the hyperviscoelastic behavior of a tire tread compound reinforced by silica and carbon black. J. Appl. Polym. Sci. 2013, 128, 1725–1731. [Google Scholar]
- Unice, K.M.; Kreider, M.L.; Panko, J.M. Use of a deuterated internal standard with pyrolysis-GC/MS dimeric marker analysis to quantify tire tread particles in the environment. Int. J. Environ. Res. Public Health 2012, 9, 4033–4055. [Google Scholar] [CrossRef]
- Seidelt, S.; Müller-Hagedorn, M.; Bockhorn, H. Description of tire pyrolysis by thermal degradation behaviour of main components. J. Anal. Appl. Pyrolysis 2006, 75, 11–18. [Google Scholar] [CrossRef]
- Choi, S.-S. Characteristics of pyrolysis pattern of styrene-butadiene rubbers with differing microstructures. J. Anal. Appl. Pyrolysis 2002, 62, 319–330. [Google Scholar] [CrossRef]
- Choi, S.-S.; Han, D.-H. Pyrolysis paths of polybutadiene depending on pyrolysis temperature. Macromol. Res. 2006, 14, 354–358. [Google Scholar] [CrossRef]










| Particle | Ratio (wt%) |
|---|---|
| Tire-road wear particles (TRWPs) | 0.5 |
| Asphalt pavement wear particles (APWPs) | 7.0 |
| Mineral particles (MPs) | 77.2 |
| Glass particles (GPs) | 11.7 |
| Glass beads (GBs) | 1.5 |
| Road paint wear particles (RPWPs) | 1.1 |
| Plant-related particles (PRPs) | 0.9 |
| Plastic particles (PPs) | 0.1 |
| Density (g/cm3) | TRWP No. | Length (µm) | Width (µm) | Aspect Ratio |
|---|---|---|---|---|
| 1.20–1.30 | L1 | 906.818 | 443.243 | 2.046 |
| L2 | 819.610 | 328.868 | 2.492 | |
| 1.40–1.50 | L3 | 2210.560 | 70.730 | 31.253 |
| Density (g/cm3) | TRWP No. | Length (µm) | Width (µm) | Aspect Ratio |
|---|---|---|---|---|
| 1.20–1.30 | M1 | 213.525 | 102.610 | 2.081 |
| M2 | 216.658 | 79.177 | 2.736 | |
| M3 | 288.630 | 101.299 | 2.849 | |
| M4 | 212.629 | 64.337 | 3.305 | |
| M5 | 388.050 | 100.524 | 3.860 | |
| 1.30–1.40 | M6 | 81.069 | 49.217 | 1.647 |
| M7 | 97.367 | 45.208 | 2.154 | |
| M8 | 207.638 | 91.421 | 2.271 | |
| M9 | 354.829 | 68.065 | 5.213 | |
| 1.40–1.50 | M10 | 142.078 | 57.064 | 2.490 |
| M11 | 744.581 | 189.547 | 3.928 | |
| 1.50–1.60 | M12 | 176.207 | 97.506 | 1.807 |
| M13 | 387.485 | 104.674 | 3.702 | |
| 1.60–1.70 | M14 | 84.341 | 69.123 | 1.220 |
| M15 | 119.108 | 68.880 | 1.729 | |
| M16 | 135.348 | 76.499 | 1.769 | |
| M17 | 155.191 | 86.731 | 1.789 | |
| M18 | 173.171 | 86.788 | 1.995 | |
| M19 | 167.619 | 69.844 | 2.400 | |
| M20 | 152.797 | 48.497 | 3.151 | |
| M21 | 166.919 | 43.192 | 3.865 |
| Density (g/cm3) | TRWP No. | Length (µm) | Width (µm) | Aspect Ratio |
|---|---|---|---|---|
| 1.30–1.40 | S1 | 75.987 | 62.774 | 1.210 |
| S2 | 84.482 | 69.276 | 1.219 | |
| S3 | 104.769 | 66.094 | 1.585 | |
| S4 | 102.466 | 58.350 | 1.756 | |
| S5 | 85.681 | 40.413 | 2.120 | |
| S6 | 89.423 | 41.858 | 2.136 | |
| S7 | 143.217 | 64.288 | 2.228 | |
| S8 | 106.022 | 42.768 | 2.479 | |
| S9 | 175.500 | 67.418 | 2.603 | |
| S10 | 132.855 | 47.826 | 2.778 | |
| S11 | 122.079 | 42.096 | 2.900 | |
| S12 | 181.297 | 51.182 | 3.542 | |
| S13 | 185.188 | 44.504 | 4.161 | |
| 1.40–1.50 | S14 | 68.380 | 51.267 | 1.334 |
| S15 | 51.300 | 36.674 | 1.399 | |
| S16 | 74.494 | 50.048 | 1.488 | |
| S17 | 70.844 | 46.010 | 1.540 | |
| S18 | 79.489 | 50.976 | 1.559 | |
| S19 | 71.347 | 45.476 | 1.569 | |
| S20 | 70.813 | 44.320 | 1.598 | |
| S21 | 74.040 | 37.798 | 1.959 | |
| S22 | 96.020 | 46.071 | 2.084 | |
| S23 | 87.544 | 41.531 | 2.108 | |
| S24 | 75.285 | 34.674 | 2.171 | |
| S25 | 95.998 | 43.113 | 2.227 | |
| S26 | 88.719 | 38.018 | 2.334 | |
| S27 | 69.131 | 26.746 | 2.585 | |
| S28 | 92.232 | 30.691 | 3.005 | |
| S29 | 87.772 | 27.016 | 3.249 | |
| S30 | 160.384 | 41.452 | 3.869 | |
| S31 | 244.556 | 62.589 | 3.907 | |
| S32 | 75.939 | 17.631 | 4.307 | |
| 1.50–1.60 | S33 | 78.593 | 53.737 | 1.463 |
| S34 | 104.537 | 70.370 | 1.486 | |
| S35 | 58.257 | 37.340 | 1.560 | |
| S36 | 110.743 | 65.747 | 1.684 | |
| S37 | 71.824 | 39.602 | 1.814 | |
| S38 | 80.917 | 41.093 | 1.969 | |
| S39 | 76.901 | 31.860 | 2.414 | |
| S40 | 93.174 | 36.110 | 2.580 | |
| S41 | 159.109 | 54.829 | 2.902 | |
| S42 | 126.799 | 42.858 | 2.959 | |
| S43 | 150.389 | 48.767 | 3.084 | |
| S44 | 112.468 | 34.436 | 3.266 | |
| S45 | 139.460 | 40.920 | 3.408 | |
| S46 | 111.306 | 32.553 | 3.419 | |
| S47 | 105.397 | 30.033 | 3.509 | |
| S48 | 121.955 | 34.548 | 3.530 | |
| S49 | 168.570 | 47.300 | 3.564 | |
| 1.60–1.70 | S50 | 150.119 | 106.791 | 1.406 |
| S51 | 100.285 | 68.619 | 1.461 | |
| S52 | 109.567 | 72.483 | 1.512 | |
| S53 | 210.149 | 131.425 | 1.599 | |
| S54 | 183.867 | 107.128 | 1.716 | |
| S55 | 99.901 | 53.580 | 1.865 | |
| S56 | 82.631 | 42.693 | 1.935 | |
| S57 | 97.152 | 48.720 | 1.994 | |
| S58 | 152.058 | 74.227 | 2.049 | |
| S59 | 107.015 | 50.644 | 2.113 | |
| S60 | 194.690 | 84.565 | 2.302 | |
| S61 | 83.901 | 35.866 | 2.339 | |
| S62 | 127.199 | 49.838 | 2.552 | |
| S63 | 123.673 | 48.075 | 2.573 | |
| S64 | 285.707 | 75.658 | 3.776 | |
| S65 | 364.601 | 91.268 | 3.995 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, U.; Choi, S.-S. Classification and Characterization of Tire-Road Wear Particles in Road Dust by Density. Polymers 2022, 14, 1005. https://doi.org/10.3390/polym14051005
Jung U, Choi S-S. Classification and Characterization of Tire-Road Wear Particles in Road Dust by Density. Polymers. 2022; 14(5):1005. https://doi.org/10.3390/polym14051005
Chicago/Turabian StyleJung, Uiyeong, and Sung-Seen Choi. 2022. "Classification and Characterization of Tire-Road Wear Particles in Road Dust by Density" Polymers 14, no. 5: 1005. https://doi.org/10.3390/polym14051005
APA StyleJung, U., & Choi, S. -S. (2022). Classification and Characterization of Tire-Road Wear Particles in Road Dust by Density. Polymers, 14(5), 1005. https://doi.org/10.3390/polym14051005