1. Introduction
The rubber industry makes remarkable contribution to the world economy, and the production of rubber from the footwear and tire industries grows annually, but it also results in a large amount of waste [
1,
2,
3]. Despite decades of research in this field, the disposal of waste rubber remains an economic, social, and environmental issue [
4]. The improper disposal of waste rubber results in a large amount of waste discarded or incinerated, which causes environmental damage and even has the risk of spreading diseases, affecting dramatically the quality of human life [
5].
Waste tire and footwear rubber are usually vulcanized, which is mainly composed of complex mixtures such as inorganic fillers, activators, accelerators, pigments, and stabilizers. When substances with low molar mass are discarded randomly in the natural environment, they may permeate the soil and the groundwater over time and seriously harm human health [
6,
7,
8,
9,
10]. At present, it is hard to reclaim vulcanized rubber because of its three-dimensional cross-linked structure network [
11]. It is therefore of great significance to come up with more effective and environmentally friendly technologies to reclaim these waste rubbers.
Generally, the existing reclamation techniques for waste rubber (WR) include microwave [
12,
13,
14], ultrasound [
15,
16], chemical modification [
17,
18,
19,
20,
21], ambient grinding [
22], cryogenic grinding [
23,
24], and biological methods [
25]. However, low yield and high energy consumption technologies are not suitable for industrial applications. Compared with the above-mentioned techniques, the thermo-mechanical reclaiming of vulcanized rubber wastes has aroused public attention on account of easy procedures and high yield as well as the use of commercially available equipment.
The mechanochemical reclaiming has the advantages of better processability and mechanical properties along with the enhanced interfacial bonding between reclaiming rubber powder and rubber. The mechanochemical reclaiming is based on mechanical shearing and a strong rise of temperature, brought by an external heat source as well as the friction between the crumb rubbers. This leads to devulcanization and the formation of shorter polymer chains by the rearrangement of the polysulfide cross-links and the degradation of carbon-carbon bonds [
1]. It is worth noting that thermo-mechanical methods with suitable temperature and filling rate can be applied to achieve devulcanization without the devulcanizing agent [
26].
A great deal of achievements have been made in the thermomechanical reclaiming of vulcanized rubber wastes. Liu and his coworker investigated the effects of mechano-chemical degradation of the crosslinked and foamed ethylene-vinyl acetate copolymer (EVA) multicomponent and multiphase waste material in a solid state by torque rheometer, and it was found that the ruptures of chemical bonds in its net and stereo-structures are caused by external mechanical energy input by the Roller rotors of the HAAKE rheometer, and the optimum mechanochemical degradation conditions are at 120–130 °C in a Roller rotor rotation speed range of 40–60 rpm for 24 min [
27]. Formela et al. investigated the reclaiming of ground tire rubber (GTR) using a corotating twin-screw extruder at different barrel temperatures (60, 120, and 180 °C). The authors observed that the increase of the barrel temperature decreases the Mooney viscosity, crosslinking density, and optimum cure time (t
90) during the revulcanization of the reclaimed GTR [
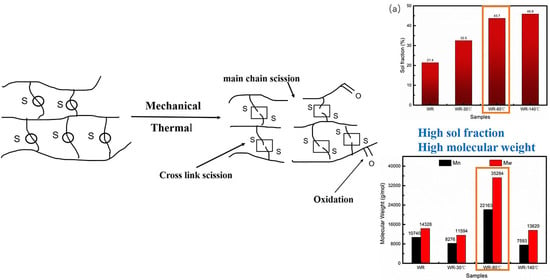
28]. Recently, Zhang et al. developed a thermal-oxidative reclamation process using a newly designed dynamic reclamation reactor, in the absence of any chemical agent, a high reclamation degree of GTR (sol fraction 66.53 wt%) was obtained at 200 °C for 20 min. The authors concluded that oxidative reclamation induced cross-link breakage and main-chain scission simultaneously, destroying the cross-linked network of the GTR, which leads to the increase of the sol fraction and decrease of the molecular weight [
29]. However, such thermal-mechanical recycle methods remain a great challenge in obtaining recycling rubber with both high molecular weight and sol content.
The pressure and grinding temperature play an important role in the grinding of the largest particles for WR. Increasing the pressure or reducing the grinding temperature would reduce the size of the largest particles by the combined breakage mechanism including abrasion, cleavage, and fracture [
30]. However, it has been recognized that the unsaturated double bonds of the main chains combined with oxygen can cause a different degree of oxidative degradation under the action of heat [
31]. Therefore, it is urgent to investigate the combination of thermal process and mechanochemical grinding.
In this work, the WR from the shoe industry was ground and reclaimed by torque rheometer, and the effects of different filling rate and grinding temperature on the structure of WR were investigated. In addition, the structure evolution and morphology of WR were also investigated in detail by using laser particle size analyzer Fourier transform infrared spectroscopy (FTIR), sol fraction analysis, gel permeation chromatography (GPC), differential scanning calorimeter (DSC), and scanning electron microscope (SEM).
4. Conclusions
In this study, the influence of different temperatures and filling rates on the structure and properties of the waste rubber from the shoe industry was investigated by means of thermo-mechanical grinding technology. The results show that the temperature and filling rate have important effects on the physical and chemical structure of WR. The thermo-mechanical method could reduce the particle size of WR. Increasing the grinding temperature causes a decrease in the sample energy consumption and Tg, while sol fraction increases. While with the increase of filling rate, the sample energy consumption and sol fraction increase and the particle size decreases. Obviously, the main chain fracture was destroyed by high grinding temperature and filling rate. Obviously, the oxidation reaction occurs during the thermal-mechanical grinding. The particle size distribution of WR after being ground can be described by Rosin’s equation. The best effect of thermal mechanical grinding is the temperature of 80 °C with filling rate of 85%. The results of sol fraction, GPC and DSC show that the crosslinking fracture and main chain fracture were induced by thermal mechanical grinding, which destroys the crosslinking network of WR, resulting in the increase of sol fraction and molecular weight. The re-vulcanization characteristics, on the whole, show a similar trend and ΔM values as pure SBR. However, the increase of MH depends on the content of RWR because residual accelerators in RWR promote the increase of the degree of crosslinking of the composites. The RWR with a high reclamation degree shows good dispersibility and reinforcement in SBR. Adding 10−40 phr RWR to SBR had no adverse effect on the mechanical properties; instead, it improves the mechanical properties of SBR. These results demonstrate that thermo-mechanical grinding under suitable condition is a cost-effective, reliable, and environmentally friendly method to recycled rubber, without any additional materials or chemical, providing some guidelines in the upgrading of waste rubber in the shoe industry.