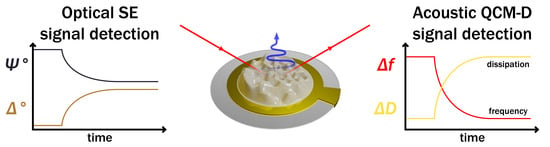
Spectroscopic Ellipsometry and Quartz Crystal Microbalance with Dissipation for the Assessment of Polymer Layers and for the Application in Biosensing
Abstract
:1. Introduction
2. Operation Principle of Spectroscopic Ellipsometry
3. Limitations of Spectroscopic Ellipsometry
4. Spectroscopic Ellipsometry for Polymers Analysis
4.1. The Application of Spectroscopic Ellipsometry for the Characterization of Optical Properties of Polymers
4.2. In Situ Spectroscopic Ellipsometry Application for the Determination of Polymers Thickness and Optical Properties
4.3. Application of Spectroscopic Ellipsometry for the Assessment of Polymers Used in Biosensing
4.4. Application of Imaging Ellipsometry for the Analysis of Polymers
5. Quartz Crystal Microbalance with Dissipation for the Assessment of Polymers
5.1. Operation Principle of Quartz Crystal Microbalance with Dissipation
5.2. Application of Polymers in QCM-D-Based Biosensors
5.3. Complementary SE/QCM-D Technique for Polymer Analysis and Biosensing
6. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramanavicius, S.; Ramanavicius, A. Conducting Polymers in the Design of Biosensors and Biofuel Cells. Polymers 2021, 13, 49. [Google Scholar] [CrossRef]
- Popov, A.; Brasiunas, B.; Damaskaite, A.; Plikusiene, I.; Ramanavicius, A.; Ramanaviciene, A. Electrodeposited Gold Nanostructures for the Enhancement of Electrochromic Properties of PANI–PEDOT Film Deposited on Transparent Electrode. Polymers 2020, 12, 2778. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.; Aukstakojyte, R.; Gaidukevic, J.; Lisyte, V.; Kausaite-Minkstimiene, A.; Barkauskas, J.; Ramanaviciene, A. Reduced Graphene Oxide and Polyaniline Nanofibers Nanocomposite for the Development of an Amperometric Glucose Biosensor. Sensors 2021, 21, 948. [Google Scholar] [CrossRef] [PubMed]
- German, N.; Popov, A.; Ramanaviciene, A.; Ramanavicius, A. Evaluation of enzymatic formation of polyaniline nanoparticles. Polymer 2017, 115, 211–216. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Charge Transfer and Biocompatibility Aspects in Conducting Polymer-Based Enzymatic Biosensors and Biofuel Cells. Nanomaterials 2021, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Ramanaviciene, A.; Plikusiene, I. Polymers in Sensor and Biosensor Design. Polymers 2021, 13, 917. [Google Scholar] [CrossRef] [PubMed]
- Ramanavičius, A.; Ramanavičienė, A.; Malinauskas, A. Electrochemical sensors based on conducting polymer—Polypyrrole. Electrochim. Acta 2006, 51, 6025–6037. [Google Scholar] [CrossRef]
- Ratautaite, V.; Topkaya, S.N.; Mikoliunaite, L.; Ozsoz, M.; Oztekin, Y.; Ramanaviciene, A.; Ramanavicius, A. Molecularly Imprinted Polypyrrole for DNA Determination. Electroanalysis 2013, 25, 1169–1177. [Google Scholar] [CrossRef]
- Baleviciute, I.; Ratautaite, V.; Ramanaviciene, A.; Balevicius, Z.; Broeders, J.; Croux, D.; McDonald, M.; Vahidpour, F.; Thoelen, R.; De Ceuninck, W.; et al. Evaluation of theophylline imprinted polypyrrole film. Synth. Met. 2015, 209, 206–211. [Google Scholar] [CrossRef]
- Ratautaite, V.; Plausinaitis, D.; Baleviciute, I.; Mikoliunaite, L.; Ramanaviciene, A.; Ramanavicius, A. Characterization of caffeine-imprinted polypyrrole by a quartz crystal microbalance and electrochemical impedance spectroscopy. Sens. Actuators B Chem. 2015, 212, 63–71. [Google Scholar] [CrossRef]
- Lu, C.-H.; Zhang, Y.; Tang, S.-F.; Fang, Z.-B.; Yang, H.-H.; Chen, X.; Chen, G.-N. Sensing HIV related protein using epitope imprinted hydrophilic polymer coated quartz crystal microbalance. Biosens. Bioelectron. 2012, 31, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, S.; Jagminas, A.; Ramanavicius, A. Advances in Molecularly Imprinted Polymers Based Affinity Sensors (Review). Polymers 2021, 13, 974. [Google Scholar] [CrossRef] [PubMed]
- Brinson, H.F.; Brinson, L.C. Characteristics, Applications and Properties of Polymers BT—Polymer Engineering Science and Viscoelasticity: An Introduction; Brinson, H.F., Brinson, L.C., Eds.; Springer: Boston, MA, USA, 2008; pp. 55–97. ISBN 978-0-387-73861-1. [Google Scholar]
- Richter, R.P.; Rodenhausen, K.B.; Eisele, N.B.; Schubert, M. Coupling Spectroscopic Ellipsometry and Quartz Crystal Micro-balance to Study Organic Films at the Solid–Liquid Interface; Springer: Cham, Switzerland, 2018; Volume 52, ISBN 9783319758954. [Google Scholar]
- Alfonso, F.S.; Zhou, Y.; Liu, E.; McGuire, A.F.; Yang, Y.; Kantarci, H.; Li, D.; Copenhaver, E.; Zuchero, J.B.; Müller, H.; et al. Label-free optical detection of bioelectric potentials using electrochromic thin films. Proc. Natl. Acad. Sci. USA 2020, 117, 17260–17268. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, E.; Müller, H.; Cui, B. Optical Electrophysiology: Toward the Goal of Label-Free Voltage Imaging. J. Am. Chem. Soc. 2021, 143, 10482–10499. [Google Scholar] [CrossRef]
- Fujiwara, H. Spectroscopic Ellipsometry: Principles and Applications; 1. Aufl.; Wiley: New York, NY, USA, 2007; ISBN 0470016086. [Google Scholar]
- Gonçalves, D.; Irene, E.A. Fundamentals and applications of spectroscopic ellipsometry. Quim. Nova 2002, 25, 794–800. [Google Scholar] [CrossRef] [Green Version]
- Irene, E. Applications of spectroscopic ellipsometry to microelectronics. Thin Solid Film. 1993, 233, 96–111. [Google Scholar] [CrossRef]
- Herman, P. Irving. In CHAPTER 9—Reflection; Optical Diagnostics for Thin Film Processing; Academic Press: San Diego, CA, USA, 1996; pp. 327–479. ISBN 978-0-12-342070-1. [Google Scholar]
- Tonda-Turo, C.; Carmagnola, I.; Ciardelli, G. Quartz Crystal Microbalance with Dissipation Monitoring: A Powerful Method to Predict the in vivo Behavior of Bioengineered Surfaces. Front. Bioeng. Biotechnol. 2018, 6, 158. [Google Scholar] [CrossRef] [PubMed]
- Karczmarczyk, A.; Haupt, K.; Feller, K.-H. Development of a QCM-D biosensor for Ochratoxin A detection in red wine. Talanta 2017, 166, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zong, Y.; Richter, R.P.; Knoll, W. Enzyme immobilization on poly(ethylene-co-acrylic acid) films studied by quartz crystal microbalance with dissipation monitoring. J. Colloid Interface Sci. 2005, 287, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung danner Schichten und zur Mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Dixon, M.C. Quartz crystal microbalance with dissipation monitoring: Enabling real-time characterization of biological mate-rials and their interactions. J. Biomol. Tech. 2008, 19, 151–158. [Google Scholar] [PubMed]
- Ramos, J.J.I.; Stahl, S.; Richter, R.P.; Moya, S.E. Water Content and Buildup of Poly(diallyldimethylammonium chloride)/Poly(sodium 4-styrenesulfonate) and Poly(allylamine hydrochloride)/Poly(sodium 4-styrenesulfonate) Polyelectrolyte Multilayers Studied by an in Situ Combination of a Quartz Crystal Microbalance with Dissipation Monitoring and Spectroscopic Ellipsometry. Macromolecules 2010, 43, 9063–9070. [Google Scholar] [CrossRef]
- Sweity, A.; Ying, W.; Ali-Shtayeh, M.S.; Yang, F.; Bick, A.; Oron, G.; Herzberg, M. Relation between EPS adherence, viscoelastic properties, and MBR operation: Biofouling study with QCM-D. Water Res. 2011, 45, 6430–6440. [Google Scholar] [CrossRef] [PubMed]
- Larsson, E.M.; Edvardsson, M.E.M.; Langhammer, C.; Zorić, I.; Kasemo, B. A combined nanoplasmonic and electrodeless quartz crystal microbalance setup. Rev. Sci. Instrum. 2009, 80, 125105. [Google Scholar] [CrossRef] [PubMed]
- Campoy-Quiles, M.; Alonso, M.I.; Bradley, D.; Richter, L. Advanced Ellipsometric Characterization of Conjugated Polymer Films. Adv. Funct. Mater. 2013, 24, 2116–2134. [Google Scholar] [CrossRef]
- Leman, D.; Kelly, M.A.; Ness, S.; Engmann, S.; Herzing, A.; Snyder, C.; Ro, H.W.; Kline, R.; Delongchamp, D.M.; Richter, L. In Situ Characterization of Polymer–Fullerene Bilayer Stability. Macromolecules 2015, 48, 383–392. [Google Scholar] [CrossRef]
- Balevicius, Z.; Ramanaviciene, A.; Baleviciute, I.; Makaraviciute, A.; Mikoliunaite, L.; Ramanavicius, A. Evaluation of intact- and fragmented-antibody based immunosensors by total internal reflection ellipsometry. Sens. Actuators B Chem. 2011, 160, 555–562. [Google Scholar] [CrossRef]
- Balevicius, Z.; Paulauskas, A.; Plikusiene, I.; Mikoliunaite, L.; Bechelany, M.; Popov, A.; Ramanavicius, A.; Ramanaviciene, A. Towards the application of Al2O3/ZnO nanolaminates in immunosensors: Total internal reflection spectroscopic ellipsometry based evaluation of BSA immobilization. J. Mater. Chem. C 2018, 6, 8778–8783. [Google Scholar] [CrossRef]
- Plikusiene, I.; Maciulis, V.; Ramanaviciene, A.; Balevicius, Z.; Buzavaite-Verteliene, E.; Ciplys, E.; Slibinskas, R.; Simanavicius, M.; Zvirbliene, A.; Ramanavicius, A. Evaluation of kinetics and thermodynamics of interaction between immobilized SARS-CoV-2 nucleoprotein and specific antibodies by total internal reflection ellipsometry. J. Colloid Interface Sci. 2021, 594, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Buzavaite-Verteliene, E.; Plikusiene, I.; Tolenis, T.; Valavičius, A.; Anulyte, J.; Ramanavicius, A.; Balevicius, Z. Hybrid Tamm-surface plasmon polariton mode for highly sensitive detection of protein interactions. Opt. Express 2020, 28, 29033–29043. [Google Scholar] [CrossRef]
- Balevicius, Z.; Talbot, J.; Tamosaitis, L.; Plikusiene, I.; Stirke, A.; Mickiene, G.; Balevicius, S.; Paulauskas, A.; Ramanavicius, A. Modelling of immunosensor response: The evaluation of binding kinetics between an immobilized receptor and structurally-different genetically engineered ligands. Sens. Actuators B Chem. 2019, 297, 126770. [Google Scholar] [CrossRef]
- Plikusiene, I.; Balevicius, Z.; Ramanaviciene, A.; Talbot, J.; Mickiene, G.; Balevicius, S.; Stirke, A.; Tereshchenko, A.; Tamosaitis, L.; Zvirblis, G.; et al. Evaluation of affinity sensor response kinetics towards dimeric ligands linked with spacers of different rigidity: Immobilized recombinant granulocyte colony-stimulating factor based synthetic receptor binding with genetically engineered dimeric analyte derivatives. Biosens. Bioelectron. 2020, 156, 112112. [Google Scholar] [CrossRef]
- Podraza, N.; Jellison, G. Ellipsometry. Encycl. Spectrosc. Spectrom. 2016, 482–489. Available online: https://www.osti.gov/biblio/1126966 (accessed on 14 February 2022). [CrossRef]
- Nestler, P.; Helm, C.A. Determination of refractive index and layer thickness of nm-thin films via ellipsometry. Opt. Express 2017, 25, 27077–27085. [Google Scholar] [CrossRef]
- Woollam, J.A. Thin Film Thickness. Available online: https://www.jawoollam.com/resources/ellipsometry-tutorial/thin-film-thickness (accessed on 14 February 2022).
- Winfield, J.M.; Donley, C.; Kim, J.-S. Anisotropic optical constants of electroluminescent conjugated polymer thin films determined by variable-angle spectroscopic ellipsometry. J. Appl. Phys. 2007, 102, 063505. [Google Scholar] [CrossRef]
- Karabiyik, U.; Mao, M.; Satija, S.K.; Esker, A.R. Determination of thicknesses and refractive indices of polymer thin films by multiple incident media ellipsometry. Thin Solid Film. 2014, 565, 72–78. [Google Scholar] [CrossRef]
- Hilfiker, J.N.; Stadermann, M.; Sun, J.; Tiwald, T.; Hale, J.S.; Miller, P.E.; Aracne-Ruddle, C. Determining thickness and refractive index from free-standing ultra-thin polymer films with spectroscopic ellipsometry. Appl. Surf. Sci. 2017, 421, 508–512. [Google Scholar] [CrossRef]
- Tompkins, H.G.; Hilfiker, J.N. Spectroscopic Ellipsometry: Practical Application to Thin Film Characterization; Momentum Press: New York, NY, USA, 2015. [Google Scholar]
- Zhang, X.; Qiu, J.; Zhao, J.; Li, X.; Liu, L. Complex refractive indices measurements of polymers in infrared bands. J. Quant. Spectrosc. Radiat. Transf. 2020, 252, 107063. [Google Scholar] [CrossRef]
- Buffon, E.; Huguenin, J.; da Silva, L.; Carneiro, P.; Stradiotto, N. Spectroscopic ellipsometry studies of an electrochemically synthesized molecularly imprinted polymer for the detection of an aviation biokerosene contaminant. React. Funct. Polym. 2020, 155, 104698. [Google Scholar] [CrossRef]
- Derkowska-Zielinska, B.; Skowronski, L.; Biitseva, A.; Grabowski, A.; Naparty, M.; Smokal, V.; Kysil, A.; Krupka, O. Optical characterization of heterocyclic azo dyes containing polymers thin films. Appl. Surf. Sci. 2017, 421, 361–366. [Google Scholar] [CrossRef]
- Bin Liu, B.; Wang, M.; He, A.Y.; Wang, X. Duplication of Photoinduced Azo Polymer Surface-Relief Gratings through a Soft Lithographic Approach. Langmuir 2006, 22, 7405–7410. [Google Scholar] [CrossRef]
- Kawata, S.; Kawata, Y. Three-Dimensional Optical Data Storage Using Photochromic Materials. Chem. Rev. 2000, 100, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Yager, K.; Barrett, C.J. Photomechanical Surface Patterning in Azo-Polymer Materials. Macromolecules 2006, 39, 9320–9326. [Google Scholar] [CrossRef]
- Czaplicki, R.; Krupka, O.; Essaidi, Z.; El-Ghayoury, A.; Kajzar, F.; Grote, J.G.; Sahraoui, B. Grating inscription in picosecond regime in thin films of functionalized DNA. Opt. Express 2007, 15, 15268–15273. [Google Scholar] [CrossRef]
- Schab-Balcerzak, E.; Flakus, H.; Jarczyk-Jedryka, A.; Konieczkowska, J.; Siwy, M.; Bijak, K.; Sobolewska, A.; Stumpe, J. Photochromic supramolecular azopolyimides based on hydrogen bonds. Opt. Mater. 2015, 47, 501–511. [Google Scholar] [CrossRef]
- Hajduk, B.; Bednarski, H.; Jarząbek, B.; Janeczek, H.; Nitschke, P. P3HT:PCBM blend films phase diagram on the base of variable-temperature spectroscopic ellipsometry. Beilstein J. Nanotechnol. 2018, 9, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Adam, G.; Munkhbat, B.; Denk, P.; Ulbricht, C.; Hrelescu, C.; Scharber, M.C. Different Device Architectures for Bulk-Heterojunction Solar Cells. Front. Mater. 2016, 3, 39. [Google Scholar] [CrossRef]
- Tzounis, L.; Gravalidis, C.; Papamichail, A.; Logothetidis, S. Enhancement of P3HT:PCBM Photovoltaic Shells Efficiency Incorporating Core-shell Au@Ag Plasmonic Nanoparticles1. Mater. Today Proc. 2016, 3, 832–839. [Google Scholar] [CrossRef]
- Holliday, S.; Ashraf, R.S.; Wadsworth, A.; Baran, D.; Yousaf, S.A.; Nielsen, C.B.; Tan, C.-H.; Dimitrov, S.D.; Shang, Z.; Gasparini, N.; et al. High-efficiency and air-stable P3HT-based polymer solar cells with a new non-fullerene acceptor. Nat. Commun. 2016, 7, 11585. [Google Scholar] [CrossRef] [Green Version]
- Beaucage, G.; Composto, R.; Stein, R.S. Ellipsometric study of the glass transition and thermal expansion coefficients of thin polymer films. J. Polym. Sci. Part B Polym. Phys. 1993, 31, 319–326. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, J.; Zin, W.-C. Thickness Dependence of the Glass Transition Temperature in Thin Polymer Films. Langmuir 2001, 17, 2703–2710. [Google Scholar] [CrossRef]
- Sharp, J.; Forrest, J.A. Dielectric and ellipsometric studies of the dynamics in thin films of isotactic poly(methylmethacrylate) with one free surface. Phys. Rev. E 2003, 67, 031805. [Google Scholar] [CrossRef] [PubMed]
- Erber, M.; Tress, M.; Mapesa, E.U.; Serghei, A.; Eichhorn, K.-J.; Voit, B.; Kremer, F. Glassy Dynamics and Glass Transition in Thin Polymer Layers of PMMA Deposited on Different Substrates. Macromolecules 2010, 43, 7729–7733. [Google Scholar] [CrossRef]
- Campoy-Quiles, M.; Etchegoin, P.; Bradley, D. Exploring the potential of ellipsometry for the characterisation of electronic, optical, morphologic and thermodynamic properties of polyfluorene thin films. Synth. Met. 2005, 155, 279–282. [Google Scholar] [CrossRef]
- Kroon, R.; Gehlhaar, R.; Steckler, T.T.; Henriksson, P.; Müller, C.; Bergqvist, J.; Hadipour, A.; Heremans, P.; Andersson, M.R. New quinoxaline and pyridopyrazine-based polymers for solution-processable photovoltaics. Sol. Energy Mater. Sol. Cells 2012, 105, 280–286. [Google Scholar] [CrossRef]
- Wang, T.; Pearson, A.J.; Dunbar, A.D.F.; Staniec, P.A.; Watters, D.C.; Coles, D.; Yi, H.; Iraqi, A.; Lidzey, D.G.; Jones, R. Competition between substrate-mediated π-π stacking and surface-mediated Tg depression in ultrathin conjugated polymer films. Eur. Phys. J. E 2012, 35, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cianci, E.; Nazzari, D.; Seguini, G.; Perego, M. Trimethylaluminum Diffusion in PMMA Thin Films during Sequential Infiltration Synthesis: In Situ Dynamic Spectroscopic Ellipsometric Investigation. Adv. Mater. Interfaces 2018, 5, 1801016. [Google Scholar] [CrossRef]
- Saftics, A.; Agócs, E.; Fodor, B.; Patkó, D.; Petrik, P.; Kolari, K.; Aalto, T.; Fürjes, P.; Horvath, R.; Kurunczi, S. Investigation of thin polymer layers for biosensor applications. Appl. Surf. Sci. 2013, 281, 66–72. [Google Scholar] [CrossRef]
- Furchner, A.; Bittrich, E.; Uhlmann, P.; Eichhorn, K.-J.; Hinrichs, K. In-situ characterization of the temperature-sensitive swelling behavior of poly(N-isopropylacrylamide) brushes by infrared and visible ellipsometry. Thin Solid Film. 2013, 541, 41–45. [Google Scholar] [CrossRef]
- Kroning, A.; Furchner, A.; Aulich, D.; Bittrich, E.; Rauch, S.; Uhlmann, P.; Eichhorn, K.-J.; Seeber, M.; Luzinov, I.; Kilbey, S.M.; et al. In Situ Infrared Ellipsometry for Protein Adsorption Studies on Ultrathin Smart Polymer Brushes in Aqueous Environment. ACS Appl. Mater. Interfaces 2015, 7, 12430–12439. [Google Scholar] [CrossRef] [PubMed]
- Aulich, D.; Hoy, O.; Luzinov, I.; Eichhorn, K.-J.; Stamm, M.; Gensch, M.; Schade, U.; Esser, N.; Hinrichs, K. In-situ IR synchrotron mapping ellipsometry on stimuli-responsive PAA-b-PS/PEG mixed polymer brushes. Phys. Status Solidi C 2010, 7, 197–199. [Google Scholar] [CrossRef]
- Heitz, T.; Drévillon, B.; Godet, C.; Bourée, J. C–H bonding of polymer-like hydrogenated amorphous carbon films investigated by in-situ infrared ellipsometry. Carbon 1999, 37, 771–775. [Google Scholar] [CrossRef]
- Maciulis, V.; Malinovskis, U.; Erts, D.; Ramanavicius, A.; Ramanaviciene, A.; Balevicius, S.; Juciute, S.; Plikusiene, I. Porous Aluminium Oxide Coating for the Development of Spectroscopic Ellipsometry Based Biosensor: Evaluation of Human Serum Albumin Adsorption. Coatings 2020, 10, 1018. [Google Scholar] [CrossRef]
- Plikusiene, I.; Maciulis, V.; Graniel, O.; Bechelany, M.; Balevicius, S.; Vertelis, V.; Balevicius, Z.; Popov, A.; Ramanavicius, A.; Ramanaviciene, A. Total internal reflection ellipsometry for kinetics-based assessment of bovine serum albumin immobilization on ZnO nanowires. J. Mater. Chem. C 2021, 9, 1345–1352. [Google Scholar] [CrossRef]
- Nabok, A.; Tsargorodskaya, A.; Mustafa, M.; Székács, A.; Starodub, N. Detection of low molecular weight toxins using optical phase detection techniques. Procedia Chem. 2009, 1, 1491–1494. [Google Scholar] [CrossRef] [Green Version]
- Arwin, H.; Poksinski, M.; Johansen, K. Total internal reflection ellipsometry: Principles and applications. Appl. Opt. 2004, 43, 3028–3036. [Google Scholar] [CrossRef] [PubMed]
- Baleviciute, I.; Balevicius, Z.; Makaraviciute, A.; Ramanaviciene, A.; Ramanavicius, A. Study of antibody/antigen binding kinetics by total internal reflection ellipsometry. Biosens. Bioelectron. 2013, 39, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Patskovsky, S.; Meunier, M.; Kabashin, A. Phase-sensitive silicon-based total internal reflection sensor. Opt. Express 2007, 15, 12523–12528. [Google Scholar] [CrossRef] [Green Version]
- Poksinski, M.; Arwin, H. Protein monolayers monitored by internal reflection ellipsometry. Thin Solid Film. 2004, 455–456, 716–721. [Google Scholar] [CrossRef]
- Le, N.C.H.; Gubala, V.; Gandhiraman, R.P.; Coyle, C.; Daniels, S.; Williams, D.E. Total internal reflection ellipsometry as a label-free assessment method for optimization of the reactive surface of bioassay devices based on a functionalized cycloolefin polymer. Anal. Bioanal. Chem. 2010, 398, 1927–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirel, G.B.; Çaykara, T. DNA immobilization on polymer-modified Si surface by controlling pH. Appl. Surf. Sci. 2009, 255, 6571–6576. [Google Scholar] [CrossRef]
- Yin, Y.; Nosworthy, N.; McKenzie, D.; Bilek, M. Ellipsometry analysis of conformational change of immobilized protein monolayer on plasma polymer surfaces. Thin Solid Film. 2011, 519, 2968–2971. [Google Scholar] [CrossRef]
- Yin, Y.; Bilek, M.M.; Fisher, K.; Guo, C.; McKenzie, D.R. An integrated solution for rapid biosensing with robust linker free covalent binding surfaces. Biosens. Bioelectron. 2013, 42, 447–452. [Google Scholar] [CrossRef] [PubMed]
- El-Sharif, H.F.; Aizawa, H.; Reddy, S.M. Spectroscopic and quartz crystal microbalance (QCM) characterisation of protein-based MIPs. Sens. Actuators B Chem. 2015, 206, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Naklua, W.; Suedee, R.; Lieberzeit, P.A. Dopaminergic receptor–ligand binding assays based on molecularly imprinted polymers on quartz crystal microbalance sensors. Biosens. Bioelectron. 2016, 81, 117–124. [Google Scholar] [CrossRef]
- Li, Q.; Quinn, J.; Wang, Y.; Caruso, F. Preparation of Nanoporous Polyelectrolyte Multilayer Films via Nanoparticle Templating. Chem. Mater. 2006, 18, 5480–5485. [Google Scholar] [CrossRef]
- Tai, D.-F.; Lin, C.-Y.; Wu, T.-Z.; Chen, L.-K. Recognition of Dengue Virus Protein Using Epitope-Mediated Molecularly Imprinted Film. Anal. Chem. 2005, 77, 5140–5143. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-T.; He, X.-W.; Li, W.-Y.; Zhang, Y.-K. Epitope molecularly imprinted polymer coated quartz crystal microbalance sensor for the determination of human serum albumin. Sens. Actuators B Chem. 2017, 246, 879–886. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Wang, C.-M.; Hsiung, K.-P.; Huang, C. Evaluation and application of conducting polymer entrapment on quartz crystal microbalance in flow injection immunoassay. Biosens. Bioelectron. 2003, 18, 937–942. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Kausaite-Minkstimiene, A.; Oztekin, Y.; Carac, G.; Voronovic, J.; German, N.; Ramanavicius, A. Visualization of red-ox proteins on the gold surface using enzymatic polypyrrole formation. Mikrochim. Acta 2011, 175, 79–86. [Google Scholar] [CrossRef]
- Plausinaitis, D.; Ratautaite, V.; Mikoliunaite, L.; Sinkevicius, L.; Ramanaviciene, A.; Ramanavicius, A. Quartz Crystal Microbalance-Based Evaluation of the Electrochemical Formation of an Aggregated Polypyrrole Particle-Based Layer. Langmuir 2015, 31, 3186–3193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fung, Y.; Sun, H.; Zhu, D.; Yao, S. Study of protein adsorption on polymer coatings surface by combining quartz crystal microbalance with electrochemical impedance methods. Sens. Actuators B Chem. 2005, 108, 933–942. [Google Scholar] [CrossRef]
- Hai, W.; Goda, T.; Takeuchi, H.; Yamaoka, S.; Horiguchi, Y.; Matsumoto, A.; Miyahara, Y. Specific Recognition of Human Influenza Virus with PEDOT Bearing Sialic Acid-Terminated Trisaccharides. ACS Appl. Mater. Interfaces 2017, 9, 14162–14170. [Google Scholar] [CrossRef] [PubMed]
- Sontimuang, C.; Suedee, R.; Canyuk, B.; Phadoongsombut, N.; Dickert, F.L. Development of a rubber elongation factor, surface-imprinted polymer–quartz crystal microbalance sensor, for quantitative determination of Hev b1 rubber latex allergens present in natural rubber latex products. Anal. Chim. Acta 2011, 687, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Bellassai, N.; Marti, A.; Spoto, G.; Huskens, J. Low-fouling, mixed-charge poly-l-lysine polymers with anionic oligopeptide side-chains. J. Mater. Chem. B 2018, 6, 7662–7673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makaraviciute, A.; Ruzgas, T.; Ramanavicius, A.; Ramanaviciene, A. Antibody fragment immobilization on planar gold and gold nanoparticle modified quartz crystal microbalance with dissipation sensor surfaces for immunosensor applications. Anal. Methods 2014, 6, 2134–2140. [Google Scholar] [CrossRef]
- Millican, J.M.; Bittrich, E.; Caspari, A.; Pöschel, K.; Drechsler, A.; Freudenberg, U.; Ryan, T.G.; Thompson, R.L.; Pospiech, D.; Hutchings, L.R. Synthesis and characterisation of a mussel-inspired hydrogel film coating for biosensors. Eur. Polym. J. 2021, 153, 110503. [Google Scholar] [CrossRef]
- Ma, H.; He, J.; Liu, X.; Gan, J.; Jin, G.; Zhou, J. Surface Initiated Polymerization from Substrates of Low Initiator Density and Its Applications in Biosensors. ACS Appl. Mater. Interfaces 2010, 2, 3223–3230. [Google Scholar] [CrossRef]
- Koenig, M.; Kasputis, T.; Schmidt, D.; Rodenhausen, K.B.; Eichhorn, K.-J.; Pannier, A.K.; Schubert, M.; Stamm, M.; Uhlmann, P. Combined QCM-D/GE as a tool to characterize stimuli-responsive swelling of and protein adsorption on polymer brushes grafted onto 3D-nanostructures. Anal. Bioanal. Chem. 2014, 406, 7233–7242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bittrich, E.; Rodenhausen, K.B.; Eichhorn, K.-J.; Hofmann, T.; Schubert, M.; Stamm, M.; Uhlmann, P. Protein adsorption on and swelling of polyelectrolyte brushes: A simultaneous ellipsometry-quartz crystal microbalance study. Biointerphases 2010, 5, 159–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rishpon, J.; Gottesfeld, S. Investigation of polypyrrole/glucose oxidase electrodes by ellipsometric, microgravimetric and electrochemical measurements. Biosens. Bioelectron. 1991, 6, 143–149. [Google Scholar] [CrossRef]
- Koenig, M.; Rodenhausen, K.B.; Schmidt, D.; Eichhorn, K.-J.; Schubert, M.; Stamm, M.; Uhlmann, P. In Situ Synthesis of Palladium Nanoparticles in Polymer Brushes Followed by QCM-D Coupled with Spectroscopic Ellipsometry. Part. Part. Syst. Charact. 2013, 30, 931–935. [Google Scholar] [CrossRef]
- Zhuang, P.; Dirani, A.; Glinel, K.; Jonas, A.M. Temperature Dependence of the Surface and Volume Hydrophilicity of Hydrophilic Polymer Brushes. Langmuir 2016, 32, 3433–3444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Rivas, M.; Guzmán, E.; Fernández-Peña, L.; Akanno, A.; Greaves, A.; Léonforte, F.; Ortega, F.; Rubio, R.G.; Luengo, G.S. Deposition of Synthetic and Bio-Based Polycations onto Negatively Charged Solid Surfaces: Effect of the Polymer Cationicity, Ionic Strength, and the Addition of an Anionic Surfactant. Colloids Interfaces 2020, 4, 33. [Google Scholar] [CrossRef]
- Niu, Y.; Jin, G. Surface modification methods to improve behavior of biosensor based on imaging ellipsometry. Appl. Surf. Sci. 2013, 281, 84–88. [Google Scholar] [CrossRef] [Green Version]
- Qi, C.; Zhu, W.; Niu, Y.; Zhang, H.G.; Zhu, G.Y.; Meng, Y.H.; Chen, S.; Jin, G. Detection of hepatitis B virus markers using a biosensor based on imaging ellipsometry. J. Viral Hepat. 2009, 16, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-H.; Jin, G. Covalent immobilization of proteins for the biosensor based on imaging ellipsometry. J. Immunol. Methods 2004, 285, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Viallat, A.; Jin, G. Vesicle adhesion visualized with total internal reflection imaging ellipsometry biosensor. Sens. Actuators B Chem. 2014, 190, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Rehfeldt, F.; Tanaka, M. Hydration Forces in Ultrathin Films of Cellulose. Langmuir 2003, 19, 1467–1473. [Google Scholar] [CrossRef]
- Ward, M.D.; Buttry, D.A. In Situ Interfacial Mass Detection with Piezoelectric Transducers. Science 1990, 249, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Rodenhausen, K.B.; Davis, R.S.; Sekora, D.; Liang, D.; Mock, A.; Neupane, R.; Schmidt, D.; Hofmann, T.; Schubert, E.; Schubert, M. The retention of liquid by columnar nanostructured surfaces during quartz crystal microbalance measurements and the effects of adsorption thereon. J. Colloid Interface Sci. 2015, 455, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.Y.; Penn, L.S.; Xi, J. Quartz crystal microbalance: Sensing cell-substrate adhesion and beyond. Biosens. Bioelectron. 2018, 99, 593–602. [Google Scholar] [CrossRef]
- Rodahl, M.; Kasemo, B. Frequency and dissipation-factor responses to localized liquid deposits on a QCM electrode. Sens. Actuators B Chem. 1996, 37, 111–116. [Google Scholar] [CrossRef]
- Höök, F.; Kasemo, B.; Nylander, T.; Fant, C.; Sott, K.; Elwing, H. Variations in Coupled Water, Viscoelastic Properties, and Film Thickness of a Mefp-1 Protein Film during Adsorption and Cross-Linking: A Quartz Crystal Microbalance with Dissipation Monitoring, Ellipsometry, and Surface Plasmon Resonance Study. Anal. Chem. 2001, 73, 5796–5804. [Google Scholar] [CrossRef]
- Yoshino, K.; Kajii, H.; Araki, H.; Sonoda, T.; Take, H.; Lee, S. Electrical and Optical Properties of Conducting Polymer—Fullerene and Conducting Polymer—Carbon Nanotube Composites, Fullerene. Sci. Technol. 1999, 7, 695–711. [Google Scholar] [CrossRef]
- Malhotra, B.D.; Chaubey, A.; Singh, S.P. Prospects of conducting polymers in biosensors. Anal. Chim. Acta 2006, 578, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Kros, A.; Nolte, R.J.M.; Sommerdijk, N.A.J.M. Poly(3,4-ethylenedioxythiophene)-based copolymers for biosensor applications. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 738–747. [Google Scholar] [CrossRef]
- Fabretto, M.; Müller, M.; Hall, C.; Murphy, P.; Short, R.D.; Griesser, H.J. In-situ QCM-D analysis reveals four distinct stages during vapour phase polymerisation of PEDOT thin films. Polymer 2010, 51, 1737–1743. [Google Scholar] [CrossRef]
- Green, R.A.; Lovell, N.H.; Poole-Warren, L.A. Cell attachment functionality of bioactive conducting polymers for neural interfaces. Biomaterials 2009, 30, 3637–3644. [Google Scholar] [CrossRef] [PubMed]
- Ramanaviciene, A.; Finkelsteinas, A.; Ramanavicius, A. Basic electrochemistry meets nanotechnology: Electrochemical prep-aration of artificial receptors based on a nanostructured conducting polymer, polypyrrole. J. Chem. Educ. 2006, 83, 1212. [Google Scholar] [CrossRef]
- Plausinaitis, D.; Sinkevicius, L.; Samukaite-Bubniene, U.; Ratautaite, V.; Ramanavicius, A. Evaluation of electrochemical quartz crystal microbalance based sensor modified by uric acid-imprinted polypyrrole. Talanta 2020, 220, 121414. [Google Scholar] [CrossRef]
- Ozcelikay, G.; Kurbanoglu, S.; Zhang, X.; Kosak Soz, C.; Wollenberger, U.; Ozkan, S.A.; Yarman, A.; Scheller, F.W. Electro-chemical MIP Sensor for Butyrylcholinesterase. Polymers 2019, 11, 1970. [Google Scholar] [CrossRef] [Green Version]
- Ramanaviciene, A.; Ramanavicius, A. Molecularly imprinted polypyrrole-based synthetic receptor for direct detection of bovine leukemia virus glycoproteins. Biosens. Bioelectron. 2004, 20, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Piletsky, S.; Canfarotta, F.; Poma, A.; Bossi, A.M.; Piletsky, S. Molecularly Imprinted Polymers for Cell Recognition. Trends Biotechnol. 2020, 38, 368–387. [Google Scholar] [CrossRef] [PubMed]
- Crystal, Q.; Method, M.; Plikusiene, I.; Ramanaviciene, A. Application of Tamm plasmon polaritons and cavity modes for biosensing in the combined spectroscopic ellipsometry and quartz crystal microbalance method. Biosensors 2021, 11, 501. [Google Scholar]





| Techniques | Polymer | Parameters Studied | Application | Ref. |
|---|---|---|---|---|
| SE | Zeonor | Optical properties | Detection of hCG using sandwich immunoassay format | [76] |
| Polyacrylamides | Layer morphology and thickness dependance on concentrations of dsDNA and incubation time | For dsDNA immobilization | [77] | |
| Plasma polymer | Refractive index, porosity, thickness | Evaluation of enzymes BLC and HRP stability | [78] | |
| Nitrogen-containing plasma polymer | Ellipsometric parameter Ψ dependance on the wavelength, layer thickness. | HRP covalent binding capacity and interaction with anti-HRP antibodies | [79] | |
| Polymer brushes containing poly(N-isopropylacrylamide) and poly(acrylic acid) | Polydispersity, grafting density, thickness in dry and wet states | HSA and FIB—adsorbing and repelling properties of nanometric thickness polymer brushes at various temperatures and pHs | [66] | |
| QCM or QCM-D | Acrylamide-based MIPs (polyacrylamide, N-hydroxymethylacrylamide, N-isopropylacrylamide) | Rebinding capacity, relative imprinting factors, selective adsorption and recognition properties | Detection of BHb | [80] |
| Acrylic acid—N-vinylpyrrolidone—N,N′-(1,2-dihydroxyethylene) bis-acrylamide based MIP | QCM response (Δf, Hz) to different concentrations of D1R | Biosensors for D1R detection (LOD = 4.3 μmol L−1, LOQ = 5.9 μmol L−1); MIP distinguish between the free receptor, receptor-dopamine complexes, and receptor-antagonist complexes | [81] | |
| Multilayer films of poly(acrylic acid), poly(allyamine hydrochloride) and silica nanoparticles | QCM response (Δf, Hz) during the formation of multilayers, effect of pH and nanoparticles size, crosslinking | Absorption of BSA | [82] | |
| acrylic acid, acrylamide, N-benzylacrylamide epitope-mediated MIP | QCM response (Δf, Hz) for different analyte concentrations | Recognition of NS1 | [83] | |
| zinc acrylate, ethylene glycol dimethacrylate based MIP | QCM response (Δf, Hz) for different concentrations of analyte, | Biosensor for HSA determination (LOD = 0.026 µg mL−1) | [84] | |
| polydopamine epitope-mediated MIP | QCM response (Δf, Hz) to different concentrations of analyte | Biosensor for HIV-1 gp41 determination (LOD = 2 ng mL−1) | [11] | |
| Polypyrrole | QCM response (Δf, Hz) to different concentrations of analyte | Immunosensor for anti-HSA determination (LOD = 0.01 mg mL−1) Immunosensor for anti-PRV determination from diluted serum samples. | [85] | |
| Polypyrrole | QCM response (Δf, Hz) during polymer formation, calculation of surface mass dencity | Evaluation of enzymatic synthesis of Ppy layer using immobilized GOx, evaluation of enzyme activity | [86] | |
| Polypyrrole | QCM response (Δf, Hz) during the electrochemical Ppy synthesis | Evaluation of electrochemical formation of aggregated Ppy particle based layer | [87] | |
| Polypyrrole based MIP | QCM response (Δf, Hz) during electrochemical formation of MIP-Ppy layer. | Biosensor for caffeine detection | [10] | |
| Polyaniline | QCM response (Δf, Hz) during electrochemical synthesis of PANI and proteins adsorption | The effect of the PANI film thickness and used doping agents on adsoption of BSA and FIB | [88] | |
| PEDOT bearing sialic acid-terminated trisaccharides | QCM response (Δf, Hz) during polymer layers formation and binding of analyte | Specific recognition of H1N1 (KD (app.) = 0.96 HAU on the 2,6- sialyllactose- modified surfaces; LOD = 0.12 HAU) | [89] | |
| Methacrylic acid–vinylpyrrolidone–dihydroxyethylene bisacrylamide based MIP | QCM response (Δf, Hz) for MIP and NIP characterization and for the analyte detection | Sensor for quantitative determination of Hev b1 (LOD = 1 μg L−1) | [90] | |
| Mixed-charge poly-L-lysine with anionic oligopeptide side-chains | QCM-D response (Δf, Hz and ΔD, 10−6) for the polymer antifouling properties analysis | BSA adsorption on the polymer surface | [91] | |
| Poly-L-lysine | QCM-D response (Δf, Hz and ΔD, 10−6) during PLL formation, gold nanoparticles and antibody fragments immobilization, abd analyte detection | Immunosensor for BLV gp51 antigen detection | [92] | |
| DMA, HEMA, GMA copolymers | SE (polymer dry thickness, in-situ polymer swelling, antibody immobilization measurements), QCM (antibody immobilization measurements) | IgG antibody immobilization on the polymer surface | [93] | |
| Complementary SE/QCM-D | Carboxylated poly(oligoethylene glycol-co-2-hydroxymethyl methacrylate) | SE (film thickness, polymer characterization). QCM response (Δf, Hz) for the polymer modification with rabbit IgG and interaction with anti-rabbit IgG | Biosensors for goat anti-rabbit IgG recognition | [94] |
| Poly-(N-isopropylacrylamide) | Complementary GE/QCM-D analysis (layer porosity and thickness measurements). | BSA adsorbtion to the polymer surface. | [95] | |
| Poly-(acrylic acid) | Complementary SE/QCM-D Polymer layer swelling in response to temperature, areal mass and viscosity measurements. | pH responsive retention and release of BSA from polymer surface. | [96] | |
| Polypyrrole | QCM response (Δf, Hz), SE response (Ψ,Δ) during PPy film growth with varying GOx concentrations. | PPy-GOx modified electrode. Manufacturing of electrochemical glucose sensor | [97] | |
| Poly(2-vinylpyridine) | Complementary SE/QCM-D in-situ analysis of Pd ion and Pd-NP enriched polymer brushes (thickness, mass, viscosity, shear modulus, proportion of metal) | Application in catalytically active nanocoatings | [98] | |
| Polymer brushes from PNIPAM, PMEO2MA, PDMA, POEGMA, PHEMA | QCM-D (Δf, Hz and ΔD, 10−6) and SE (Ψ,Δ, thickness, refractive index) response during polymer brush swelling in response to temperature. | Surface and volume hydrofilicity determination for polymer brushes as a function of temperature. Explanation of PHEMA brush antifouling properties. Further application to hydrogels. | [99] | |
| PDADMAC, CHI, JR 400 | QCM-D and SE thickness measurements during polycation adsorption onto negatively charged surfaces, effects of the polymer concentration, charge density, chemical nature, ionic strength of the solution and the addition of a surfactant, hydration level | Electrostatically-driven enhanced polymer. deposition | [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plikusiene, I.; Maciulis, V.; Ramanavicius, A.; Ramanaviciene, A. Spectroscopic Ellipsometry and Quartz Crystal Microbalance with Dissipation for the Assessment of Polymer Layers and for the Application in Biosensing. Polymers 2022, 14, 1056. https://doi.org/10.3390/polym14051056
Plikusiene I, Maciulis V, Ramanavicius A, Ramanaviciene A. Spectroscopic Ellipsometry and Quartz Crystal Microbalance with Dissipation for the Assessment of Polymer Layers and for the Application in Biosensing. Polymers. 2022; 14(5):1056. https://doi.org/10.3390/polym14051056
Chicago/Turabian StylePlikusiene, Ieva, Vincentas Maciulis, Arunas Ramanavicius, and Almira Ramanaviciene. 2022. "Spectroscopic Ellipsometry and Quartz Crystal Microbalance with Dissipation for the Assessment of Polymer Layers and for the Application in Biosensing" Polymers 14, no. 5: 1056. https://doi.org/10.3390/polym14051056
APA StylePlikusiene, I., Maciulis, V., Ramanavicius, A., & Ramanaviciene, A. (2022). Spectroscopic Ellipsometry and Quartz Crystal Microbalance with Dissipation for the Assessment of Polymer Layers and for the Application in Biosensing. Polymers, 14(5), 1056. https://doi.org/10.3390/polym14051056