One-Pot Method to Synthesize Silver Nanoparticle-Modified Bamboo-Based Carbon Aerogels for Formaldehyde Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bamboo-Based Cellulose Carbon Aerogel (BCA)
2.3. Characterization
2.4. Breakthrough Curves’ Measurement by HCHO
2.5. Adsorption Capacity of HCHO
3. Results and Discussion
3.1. Characterization of Carbon Aerogels
3.2. Adsorption Performance
3.2.1. Adsorption Process
3.2.2. Adsorption Thermodynamics
3.2.3. Breakthrough Curves
3.2.4. Comparison of Adsorption Performance of Different Ag Loaded Materials
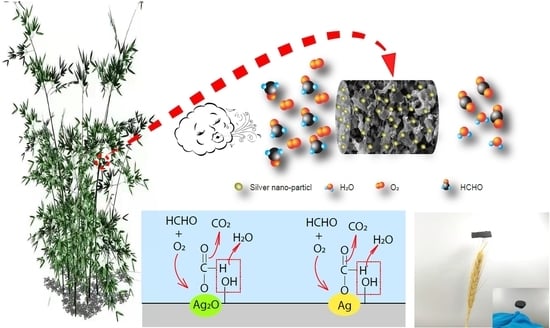
3.2.5. Mechanism Analysis of HCHO Adsorption on Ag/BCAs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salthammer, T.; Mentese, S.; Marutzky, R. Formaldehyde in the indoor environment. Chem. Rev. 2010, 110, 2536. [Google Scholar] [CrossRef]
- Rovira, J.; Roig, N.; Nadal, M.; Schuhmacher, M.; Domingo, J.L. Human health risks of formaldehyde indoor levels: An issue of concern. J. Environ. Sci. Health Part A 2016, 51, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Hadei, M.; Hopke, P.K.; Rafiee, M.; Rastkari, N.; Yarahmadi, M.; Kermani, M.; Shahsavani, A. Indoor and outdoor concentrations of BTEX and formaldehyde in Tehran, Iran: Effects of building characteristics and health risk assessment. Environ. Sci. Pollut. Res. 2018, 25, 27423. [Google Scholar]
- Vikranta, K.; Choa, M.; Khanb, A.; Kim, K.; Ahn, W.; Kwon, E.E. Adsorption properties of advanced functional materials against gaseous formaldehyde. Environ. Res. 2019, 178, 106872. [Google Scholar] [CrossRef]
- Delikhoon, M.; Fazlzadeh, M.; Sorooshian, A.; Baghani, A.N.; Golaki, M.; Ashournejad, Q.; Barkhordari, A. Characteristics and health effects of formaldehyde and acetaldehyde in an urban area in Iran. Environ. Pollut. 2018, 241, 938. [Google Scholar] [CrossRef]
- Darynova, Z.; Torkmahalleh, M.A.; Abdrakhmanov, T.; Sabyrzhan, S.; Sagynov, S.; Hopke, P.K.; Kushta, J. SO2 and HCHO over the major cities of Kazakhstan from 2005 to 2016: Infuence of political, economic and industrial changes. J. Sci. Rep. 2020, 10, 12635. [Google Scholar] [CrossRef]
- Krzyzanowski, M.; Quackenboss, J.J.; Lebowitz, M.D. Chronic respiratory effects of indoor formaldehyde exposure. Environ. Res. 1990, 52, 117. [Google Scholar] [CrossRef]
- Li, L.; Tian, S.; Jiang, J.; Xu, S.; Yong, W. Differences in purifying and resistance tolerance ability of scindapsus and chlorophytum to formaldehyde pollution. Air Qual. Atmos. Health 2020, 13, 501. [Google Scholar] [CrossRef]
- Wang, L.; Sheng, Q.; Zhang, Y.; Xu, J.; Zhang, H.; Zhu, Z. Tolerance of fifteen hydroponic ornamental plant species to formaldehyde stress. Environ. Pollut. 2020, 265, 115003. [Google Scholar] [CrossRef]
- Han, K.T.; Ruan, L.W. Effects of indoor plants on air quality: A systematic review. Environ. Sci. Pollut. Res. 2020, 27, 16019. [Google Scholar] [CrossRef]
- Diltemiz, S.E.; Ecevit, K. High-performance formaldehyde adsorption on CuO/ZnO composite nanofiber coated QCM sensors. J. Alloys Compd. 2019, 783, 608. [Google Scholar] [CrossRef]
- Shalbafan, A.; Hassannejad, H.; Rahmaninia, M. Formaldehyde adsorption capacity of chitosan derivatives as bio-adsorbents for wood-based panels. Int. J. Adhes. Adhes. 2020, 102, 102669. [Google Scholar] [CrossRef]
- Qina, Y.; Wanga, Z.; Jiangb, J.; Xing, L.; Kai, W. One-step fabrication of TiO2/Ti foil annular photoreactor for photocatalytic degradation of formaldehyde. Chem. Eng. J. 2020, 394, 124917. [Google Scholar] [CrossRef]
- Huanga, Q.; Hua, Y.; Pei, Y.; Zhang, J.; Fu, M. In situ synthesis of TiO2@NH2-MIL-125 composites for use in combined adsorption and photocatalytic degradation of formaldehyde. Appl. Catal. B Environ. 2019, 259, 118106. [Google Scholar] [CrossRef]
- Dou, H.; Long, D.; Rao, X.; Zhang, Y.; Qin, Y.; Pan, F.; Wu, K. Photocatalytic degradation kinetics of gaseous formaldehyde flow using TiO2 nanowires. ACS Sustain. Chem. Eng. 2019, 7, 4456. [Google Scholar] [CrossRef]
- Dhal, G.C.; Dey, S.; Prasad, R.; Mohan, D. Simultaneous elimination of soot and NOX through silver-barium based catalytic materials. Bull. Chem. React. Eng. Catal. 2017, 12, 71–80. [Google Scholar] [CrossRef]
- Daviesa, C.; Thompson, K.; Cooper, A.; Golunski, S.; Taylor, S.H.; Macias, M.B.; Doustdar, O.; Tsolakis, A. Simultaneous removal of NOx and soot particulate from diesel exhaust by in-situ catalytic generation and utilisation of N2O. Appl. Catal. B Environ. 2020, 271, 117627. [Google Scholar] [CrossRef]
- Xu, G.; Ma, J.; He, G.; Yu, Y.; He, H. An alumina-supported silver catalyst with high water tolerance for H2 assisted C3H6-SCR of NOx. Appl. Catal. B Environ. 2017, 207, 60. [Google Scholar] [CrossRef]
- Qu, Z.; Cheng, M.; Huang, W.; Xinhe, B. Formation of subsurface oxygen species and its high activity toward CO oxidation over silver catalysts. J. Catal. 2005, 229, 446. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, Z.; Li, X.; Wen, M.; Quan, X.; Ma, D.; Wu, J. Studies of silver species for low-temperature CO oxidation on Ag/SiO2 catalysts. J. Sep. Purif. Technol. 2010, 72, 395. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C. Applications of silver nanocatalysts for low-temperature oxidation of carbon monoxide. Inorg. Chem. Commun. 2019, 110, 107614. [Google Scholar] [CrossRef]
- Van Hoof, A.J.F.; Hermans, E.A.R.; Van Bavel, A.P.; Friedrich, H.; Hensen, E.J. Structure Sensitivity of Silver-Catalyzed Ethylene Epoxidation. ACS Catal. 2019, 9, 9829. [Google Scholar] [CrossRef]
- van Hoof, A.J.F.; van der Poll, R.C.J.; Friedrich, H.; Hensen, E.J.M. Dynamics of silver particles during ethylene epoxidation. Appl. Catal. B Environ. 2020, 272, 118983. [Google Scholar] [CrossRef]
- Harris, J.W.; Bhan, A. Kinetics of chlorine deposition and removal over promoted silver catalysts during ethylene epoxidation. J. Catal. 2019, 380, 318. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Pan, X.; Cortie, D.; Huang, X.; Yi, Z. Photocatalytic oxidation of methane over silver decorated zinc oxide nanocatalysts. Nat. Commun. 2016, 7, 12273. [Google Scholar] [CrossRef] [Green Version]
- Bao, X.; Muhler, M.; Schlögl, R.; Ertl, G. Oxidative coupling of methane on silver catalysts. Catal Lett. 1995, 32, 185. [Google Scholar] [CrossRef]
- Shimizu, K.I.; Miyamoto, Y.; Satsuma, A. Silica-supported silver nanoparticles with surface oxygen species as a reusable catalyst for alkylation of arenes. ChenCatChem 2010, 2, 84. [Google Scholar] [CrossRef]
- Batalha, L.A.R.; Colodette, J.L.; Gomide, J.L.; Barbosa, L.C.A.; Maltha, C.R.A.; Gomes, F.J.B. Dissolving pulp production from bamboo. Bioresources 2012, 7, 640. [Google Scholar]
- de Falco, G.; Barczak, M.; Montagnaro, F.; Bandosz, T.J. A new generation of surface active carbon textiles as reactive adsorbents of indoor formaldehyde. Appl. Mater. Interfaces 2018, 10, 8066. [Google Scholar] [CrossRef]
- Garibo, D.; Borbón-Nuñez, H.A.; de León, J.N.D. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci. Rep. 2020, 10, 12805. [Google Scholar] [CrossRef]
- Xu, J.; Han, X.; Liu, H. Synthesis and optical properties of silver nanoparticles stabilized by gemini surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2006, 273, 179. [Google Scholar] [CrossRef]
- Zhao, W.; Luo, L.; Wu, X.; Chen, T.; Li, Z.; Zhang, Z.; Rao, J.; Fan, M. Facile and low-cost heteroatom-doped activated biocarbons derived from fir bark for electrochemical capacitors. Wood Sci. Technol. 2019, 53, 227. [Google Scholar] [CrossRef]
- Xua, G.; Wang, L.; Liu, J.; Wu, J. FTIR and XPS analysis of the changes in bamboo chemical structure decayed by white-rot and brown-rot fungi. Appl. Surf. Sci. 2013, 280, 799. [Google Scholar] [CrossRef]
- Pircher, N.; Carbajal, L.; Schimper, C.; Bacher, M.; Rennhofer, H.; Nedelec, J.; Lichtenegger, H.C.; Rosenau, T.; Liebner, F. Impact of selected solvent systems on the pore and solid structure of cellulose aerogels. Cellulose 2016, 23, 1949. [Google Scholar] [CrossRef] [Green Version]
- Zaman, A.; Huang, F.; Jiang, M.; Wei, W.; Zhou, Z. Preparation, Properties, and Applications of Natural Cellulosic Aerogels: A Review. Energy Built Environ. 2020, 1, 60. [Google Scholar] [CrossRef]
- Rengga, W.D.P.; Chafidz, A.; Sudibandriyo, M.; Nasikin, M.; Abasaeed, A.E. Silver nano-particles deposited on bamboo-based activated carbon for removal of formaldehyde. J. Environ. Chem. Eng. 2017, 5, 1657. [Google Scholar] [CrossRef]
- Rojas, F.; Kornhauser, I.; Felipe, C.; Esparza, J.M.; Cordero, S.; Domínguez, A.; Riccardo, J.L. Capillary condensation in heterogeneous mesoporous networks consisting of variable connectivity and pore-size correlation. Phys. Chem. Chem. Phys. 2002, 4, 2346. [Google Scholar] [CrossRef]
- Tounsadi, H.; Khalidi, A.; Machrouhi, A.; Farnane, M.; Elmoubarki, R.; Elhalil, A.; Sadiq, M.; Barka, N. Highly efficient activated carbon from Glebionis coronaria L. biomass: Optimization of preparation conditions and heavy metals removal using experimental design approach. Environ. Chem. Eng. 2016, 4, 4549. [Google Scholar] [CrossRef]
- Changa, S.; Hub, S.; Shiue, A.; Lee, P.; Leggett, G. Adsorption of silver nano-particles modified activated carbon filter media for indoor formaldehyde removal. Chem. Phys. Lett. 2020, 757, 137864. [Google Scholar] [CrossRef]
- VanOsdell, D.W.; Owen, M.K.; Jaffe, L.B. VOC removal at low contaminant concentrations using granular activated carbon. Air Waste Manag. Assoc. 1996, 46, 883. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, S.B.; Jamal, A.; Faisal, M.; Asiri, A.M. Fabrication of highly sensitive acetone sensor based on sonochemically prepared as-grown Ag2O nanostructures. Chem. Eng. J. 2012, 192, 122. [Google Scholar] [CrossRef]
- Hu, S.C.; Chen, Y.C.; Lin, X.Z.; Shiue, A.; Huang, P.; Chen, Y.; Chang, S.; Tseng, C.; Zhou, B. Characterization and adsorption capacity of potassium permanganate used to modify activated carbon filter media for indoor formaldehyde removal. Environ. Sci. Pollut. Res. 2018, 25, 28525. [Google Scholar] [CrossRef]
- Rengga, W.D.P.; Sudibandriyo, M.; Nasikin, M. Adsorption of Low-Concentration Formaldehyde from Air by Silver and Copper Nano-Particles Attached on Bamboo-Based Activated Carbon. Int. J. Chem. Eng. Appl. 2013, 4, 332. [Google Scholar] [CrossRef] [Green Version]
- Lara-Ibeas, I.; Megías-Sayago, C.; Louis, B.; le Calvé, S. Adsorptive removal of gaseous formaldehyde at realistic concentrations. J. Environ. Chem. Eng. 2020, 8, 103986. [Google Scholar] [CrossRef]
- Fu, C.; Chen, T.; Liang, W.; Cai, J.; Xiao, T.; Song, Y.; Odom, T.; Xu, H. Formaldehyde Gas Adsorption in High-Capacity Silver-Nanoparticle-Loaded ZIF-8 and UiO-66 Frameworks. ChemistrySelect 2020, 5, 5987–5992. [Google Scholar] [CrossRef]
- Liu, S.; Shan, Y.; Chen, L.; Boury, B.; Huang, L.; Xiao, H. Probing nanocolumnar silver nanoparticle/zinc oxide hierarchical assemblies with advanced surface plasmon resonance and their enhanced photocatalytic performance for formaldehyde removal. Appl. Organometal Chem. 2019, 33, 5209. [Google Scholar] [CrossRef]
- Ma, L.; Seo, C.Y.; Chen, X.; Li, J.; Schwank, J.W. Sodium-promoted Ag/CeO2 nanospheres for catalytic oxidation of formaldehyde. Chem. Eng. J. 2018, 350, 419. [Google Scholar] [CrossRef]
- Ma, L.; Wang, D.; Li, J.; Bai, B.; Fu, L.; Li, Y. Ag/CeO2 nanospheres: Efficient catalysts for formaldehyde oxidation. Appl. Catal. B Environ. 2014, 148, 36. [Google Scholar] [CrossRef]
- Chen, D.; Qu, Z.; Shen, S.; Li, X.; Shi, Y.; Wang, Y.; Fu, Q.; Wu, J. Comparative studies of silver based catalysts supported on different supports for the oxidation of formaldehyde. Catal. Today 2011, 175, 338. [Google Scholar] [CrossRef]










| Sample | SBET (m2/g) | Dpore (nm) | Vpore (cm3/g) |
|---|---|---|---|
| Cellulose aerogel | 111.27 | 10.15 | 0.28 |
| BCA | 324.99 | 4.92 | 0.40 |
| 1% Ag/BCA | 329.97 | 4.25 | 0.35 |
| 3% Ag/BCA | 359.29 | 3.24 | 0.35 |
| 5% Ag/BCA | 394.20 | 3.54 | 0.29 |
| Model | Parameter | BCA | 1%Ag/BCA | 3%Ag/BCA | 5%Ag/BCA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 ppm | 50 ppm | 70 ppm | 25 ppm | 50 ppm | 70 ppm | 25 ppm | 50 ppm | 70 ppm | 25 ppm | 50 ppm | 70 ppm | ||
| Pseudo 1st order kinetic | K1 (min−1) | 0.026 | 0.080 | 0.095 | 0.021 | 0.065 | 0.080 | 0.019 | 0.058 | 0.072 | 0.013 | 0.040 | 0.052 |
| qe (g/g) | 0.007 | 0.007 | 0.006 | 0.014 | 0.018 | 0.018 | 0.031 | 0.022 | 0.019 | 0.038 | 0.027 | 0.024 | |
| R2 | 0.966 | 0.966 | 0.968 | 0.988 | 0.987 | 0.988 | 0.951 | 0.950 | 0.951 | 0.943 | 0.944 | 0.954 | |
| Pseudo 2nd order kinetic | K2 (g/g·min) | 5.555 | 17.31 | 20.27 | 2.274 | 5.407 | 6.503 | 0.915 | 3.932 | 5.531 | 0.459 | 1.956 | 2.925 |
| qe (g/g) | 0.008 | 0.008 | 0.007 | 0.015 | 0.019 | 0.020 | 0.033 | 0.023 | 0.020 | 0.042 | 0.029 | 0.026 | |
| R2 | 0.991 | 0.991 | 0.991 | 0.999 | 0.997 | 0.999 | 0.991 | 0.991 | 0.992 | 0.997 | 0.998 | 0.990 | |
| Elovich | α (g/g·min) | 0.005 | 0.016 | 0.006 | 0.005 | 0.022 | 0.025 | 0.008 | 0.018 | 0.018 | 0.004 | 0.007 | 0.009 |
| β (g/g) | 998.9 | 1037 | 938.7 | 503.8 | 395.2 | 386.3 | 220.2 | 314.0 | 356.0 | 150.6 | 215.4 | 248.9 | |
| R2 | 0.984 | 0.983 | 0.992 | 0.976 | 0.975 | 0.977 | 0.996 | 0.996 | 0.996 | 0.997 | 0.997 | 0.992 | |
| Adsorbent | Langmuir Model Parameters | ||
|---|---|---|---|
| qmax (mg/g) | KL | R2 | |
| BCA | 7.953 | 4.783 | 0.997 |
| 1% Ag/BCA | 21.56 | 0.0757 | 0.983 |
| 3% Ag/BCA | 23.56 | 0.3504 | 0.987 |
| 5% Ag/BCA | 26.75 | 0.8314 | 0.987 |
| Adsorbent | Adsorbent Type | SBET (m2/g) | Vpore(cm3/g) | Initial HCHO Concentration (ppm) | Adsorption Capacity of HCHO (mg/g) | Ref. |
|---|---|---|---|---|---|---|
| Ag-AC | Activated carbon | 685 | - | 349.9 | 0.51 | [43] |
| 0.001 M Ag-AC | Activated carbon | 1145 | 0.66 | 0.5 | 0.47 | [39] |
| HKUST-1 | MOF | 1733 | 0.89 | 0.164 | 0.50 | [44] |
| 2.5 wt%-Ag NPs@ZIF-8 | Zeolite | 1190 | 0.64 | 1.41 | 2.27 | [45] |
| Ag/ZnO-5 | ZnO | 8 | 0.12 | 10 | 12.76 | [46] |
| Ag-Na/CeO2-N | CeO2 | 92 | 0.17 | 970 | 0.200 | [47] |
| Ag/BCA | Aerogel | 394 | 0.29 | 25 | 42.00 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, W.; Yang, C.; Luo, S.; Lin, X.; Tang, M.; Zheng, R.; Lian, D.; Luo, X. One-Pot Method to Synthesize Silver Nanoparticle-Modified Bamboo-Based Carbon Aerogels for Formaldehyde Removal. Polymers 2022, 14, 860. https://doi.org/10.3390/polym14050860
Jing W, Yang C, Luo S, Lin X, Tang M, Zheng R, Lian D, Luo X. One-Pot Method to Synthesize Silver Nanoparticle-Modified Bamboo-Based Carbon Aerogels for Formaldehyde Removal. Polymers. 2022; 14(5):860. https://doi.org/10.3390/polym14050860
Chicago/Turabian StyleJing, Wenxiang, Chai Yang, Shuang Luo, Xiaoyan Lin, Min Tang, Renhong Zheng, Dongming Lian, and Xuegang Luo. 2022. "One-Pot Method to Synthesize Silver Nanoparticle-Modified Bamboo-Based Carbon Aerogels for Formaldehyde Removal" Polymers 14, no. 5: 860. https://doi.org/10.3390/polym14050860
APA StyleJing, W., Yang, C., Luo, S., Lin, X., Tang, M., Zheng, R., Lian, D., & Luo, X. (2022). One-Pot Method to Synthesize Silver Nanoparticle-Modified Bamboo-Based Carbon Aerogels for Formaldehyde Removal. Polymers, 14(5), 860. https://doi.org/10.3390/polym14050860