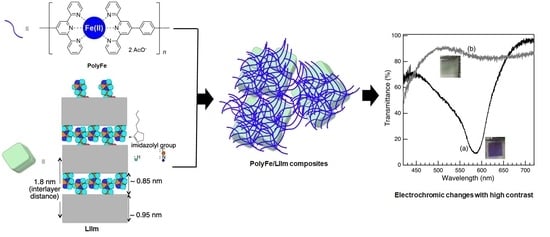
3.1. Structural Characterization
To discuss the relationship between the complexed structure and EC properties of nanocomposites, we first performed SEM and XRD. The SEM images revealed different morphologies and particle sizes among the polyFe/LIIm composites (
Figure 1). Large particles with sizes of 15–30 μm (an average size of 24 μm) were observed for the polyFe/LIIm composite-3/0.1 (
Figure 1a). A large portion of polyFe was spread on the Si wafer used for SEM observations (
Figure S1a, Supplementary Information (SI)). Agglomerates were partially observed for polyFe only (
Figure S1b, SI). The sizes of pure polyFe agglomerates were 3–175 μm (an average size of 40 μm). The surface was bumps and dips for the pure polyFe agglomerates (
Figure S1b, SI). The SEM image for the polyFe/LIIm composite-3/0.1 (
Figure 1a) demonstrated a relatively angular shape and smoother surface with crimps-like draped curtain and without the bumps and dips compared with the pure polyFe agglomerates (
Figure S1b, SI). The differences in morphologies between polyFe/LIIm composite-3/0.1 and pure polyFe could be attributed to combining polyFe with LIIm, although LIIm was rarely observed for the polyFe/LIIm composite-3/0.1.
The SEM images of LIIm demonstrated layered morphologies with a size range of 60–300 μm and with an average particle size of 130 μm that comprised stacking lamella with sizes of 26–140 μm (
Figure S2a, SI). An SEM image for LIIm with higher magnification (
Figure S2b, SI) demonstrated smaller planular particles with an average size of 290 nm (120–830 nm) stacked in the stacking direction and without order in the lateral direction to form layered morphologies.
The surface seems to be smoother for polyFe/LIIm composite-3/0.1 (
Figure 1a) than that for LIIm only (
Figure S2a,b, SI). The SEM images of LIIm only (
Figure S2a,b, SI) demonstrated a rough surface because of the stacking of LIIm smaller planular particles.
For the polyFe/LIIm composite-3/0.5, the SEM images revealed aggregations, such as sliding layered aggregates sized at several micrometers (
Figure 1b). An SEM image of the polyFe/LIIm composite-3/0.5 with a higher magnification (
Figure S3a, SI) demonstrated that the smaller planular particles with an average size of 342 nm (from 252 to 420 nm) were stacked in the stacking direction and without order in a lateral direction to form the sliding layered morphologies. Each of the smaller planular particles in the polyFe/LIIm composite-3/0.5 demonstrated slightly rounded edges and a smoother surface compared with those of the LIIm only (
Figure S2b, SI).
PolyFe/LIIm composites-3/1 and 3/5 demonstrated disordered agglomerates (
Figure 1c,d). The polyFe/LIIm composite-3/5 had larger agglomerates with sizes of 1–13 μm (an average size of 5.7μm) than polyFe/LIIm composites-3/0.5 and 3/1 with sizes of 1–8 μm (an average size of 3 μm). The images of agglomerates for polyFe/LIIm composite-3/1 (
Figure 1c) were more disordered than the polyFe/LIIm composites-3/0.5 and 3/5 (
Figure 1b,d). For the polyFe/LIIm composite-3/1, the disordered agglomerates comprised smaller planular particles with an average size of 240 (from 70–570) nm (
Figure S4, SI). The SEM image (
Figure 1d and
Figure S3b, SI) shows that the large agglomerates comprised layered aggregates with sizes of 0.5–5 μm (an average size of 1 μm), and the layered aggregates comprised smaller planular particles with an average size of 270 (80–560) nm for polyFe/LIIm composite-3/5. Because the agglomerates were disordered for the polyFe/LIIm composites-3/1 and 5, the thicknesses of the smaller planular particles were relatively easily observed. The average thicknesses were 24 (6–58) and 18 (8–36) nm for polyFe/LIIm composites-3/1 and 5, respectively. The SEM images of polyFe/LIIm composite-3/1 and 5 (
Figure 1c,d and
Figure S3b, SI) demonstrated a smoother surface and slightly rounded edges compared with those of the LIIm only (
Figure S2, SI).
SEM observations demonstrated well-ordered larger layered aggregations with an average size of 1.8 μm (0.8–3 μm) and large particles with morphologies similar to those seen in polyFe/LIIm-3/0.1 (
Figure 1a), but with smaller sizes of ~5 μm for the polyFe/LIIm composite-3/5 (not shown). Thus, SEM studies indicated the polyFe/LIIm-3/5 was inhomogeneous.
SEM observations demonstrated that the morphologies of polyFe/LIIm composites differed from both pure polyFe and LIIm. The morphologies varied with polyFe/LIIm ratios. The polyFe/LIIm composite-3/1 exhibited the most disordered agglomerates among all composites. The surface was smoother, and the edges were slightly rounded for the polyFe/LIIm composites compared to those of the LIIm only. The surfaces of the polyFe/LIIm composites and LIIm alone would have different compositions and structures.
The XRD peak was observed at a low angle (d = 1.8 nm) for LIIm (
Figure 2e) and is attributed to the interlayer distance, including the layer thickness and gallery height between the inorganic moieties of LIIm (
Scheme 1) [
30]. The XRD peak was not observed for the polyFe/LIIm composite-3/0.1 (
Figure 2a) and barely observed for the polyFe/LIIm composite-3/0.5 (
Figure 2b). The XRD peak was observed for the polyFe/LIIm composites-3/1 and 5. The XRD peak was not shifted for the composites compared with LIIm only. The gallery height (Δd) between the inorganic moieties of LIIm can be estimated at 0.85 nm by assuming that the thickness of the inorganic moiety is the same as the 0.95 nm [
33] thickness of the 2:1 layer smectites. The theoretical thickness of polyFe was calculated using MM3 as 0.9 nm [
27]. It is difficult for the polyFe to be intercalated in the interlayer with a distance of 1.8 nm. The XRD peak weakened as the ratios of LIIm to polyFe decreased. The XRD peak intensity decreased more significantly than expected based on the reduction in the ratios of LIIm in polyFe/LIIm composites. Two reasons are proposed for the reduction in the intensity of the XRD peak with decreasing ratios of LIIm to polyFe in the composites: (1) the contents of LIIm were extremely low in the composites to indicate the XRD peak, and (2) there were no layered aggregates in the composites with lower LIIm contents (polyFe/LIIm composites-3/0.1 and 0.5). Stronger scatterings were observed in the lowest angle region for polyFe/LIIm composites-3/0.1, 3/1, and 3/0.5.
These results show that most polyFe is not intercalated in the interlayer space. SEM observations demonstrated a smoother surface and slightly rounded edges for polyFe/LIIm composites compared to those of LIIm only (
Figure 1). Based on these experimental results, one can speculate that amorphous polyFe coats the smaller planular particles, layered aggregates, and disordered agglomerates of LIIm in polyFe/LIIm composites (
Scheme 2). Because of this coating, it was more difficult to recognize the layered aggregates and smaller planular particles of LIIm with increasing ratios of polyFe to LIIm.
The observed XRD peaks with a d value of 1.8 nm (
Figure 2c,d) show that LIIm layers were stacked in an order in the stacking direction in the smaller planular particles in polyFe/LIIm composites-3/1 and 5 at least in the major part. As described above, the disordered agglomerates and layered aggregates comprised smaller planular particles with average sizes of 240 and 270 nm and average thicknesses of 24 (6–58) and 18 (8–36) nm for polyFe/LIIm composites-3/1 and 5 (
Figure 1c,d). One smaller planular particle comprised stacking 14 (4–33) and 11 (5–21) LIIm layers on average (ranges) for polyFe/LIIm composites-3/1 and 5, respectively, based on the average thicknesses and an interlayer distance of 1.8 nm (
Figure 2). It is possible that higher scattering may be attributed to the partially exfoliated LIIm.
As per SEM images, the agglomerates of polyFe/LIIm composites-3/1 and 5 were larger and more disordered than aggregates of polyFe/LIIm composite-3/0.5. These variable morphologies are related to differences between the preparation media with different concentrations, as discussed below. For preparing polyFe/LIIm composites-3/0.5, 3/1, and 3/5, the LIIm dispersions in MeOH were not transparent. With MeOH, LIIm is minimally swollen and exfoliated. Accordingly, we may assume that most of LIIm’s smaller planular particles comprising stacking LIIm layers will not be exfoliated in the preparation media. In the preparation medium with a dilute concentration of LIIm for polyFe/LIIm composite-3/0.5, the dispersed smaller planular particles would be covered by polyFe and then simultaneously gather to form the reasonably ordered layered aggregates. Because the content of LIIm (smaller planular particles and aggregates) in the preparation medium was high, the agglomerates for polyFe/LIIm composites-3/1 and 3/5 were larger and more disordered than the polyFe/LIIm composite-3/0.5. Because the content of the polyFe/LIIm composite-3/5 preparation medium was high, in the beginning, the layered aggregates of LIIm did not entirely collapse into smaller planular particles. Therefore, until the stirring was completed in the preparation operation, the layered aggregates still existed. In the preparation technique for polyFe/LIIm composite-3/5, the layered aggregates of LIIm would congregate in huge agglomerates, resulting in more organized morphologies in SEM images than in the polyFe-LIIm composite-3/1.
Although many composites comprising polymers and inorganic materials have been reported, there have been few reports of composites of polymers coating inorganic materials without a special process. Inorganic particles were dispersed in the polymer matrices in many of the reported composites [
31]. We consider polyFe could coat the smaller planular particles, aggregates, and agglomerates of the negatively charged LIIm to combine in polyFe/LIIm composites with such an interesting feature because polyFe is positively charged.
In our previous study, polyFe was intercalated in the interlayer space of saponite to generate a saponite-polyFe composite [
27]. While preparing the saponite-polyFe composite, saponite is dispersed in water. In water, saponite can swell and exfoliate. For this study, LIIm was diffused in MeOH. Because the imidazolyl group lies in the interlayer gap and is anchored to the layer of LIIm, it rarely swells and exfoliates in MeOH. Consequently, polyFe is rarely intercalated in the interlayer region of LIIm, resulting in polyFe/LIIm composites with an intriguing phenomenon that differs from clay-polyFe composites previously reported. The possibility of exfoliation will be discussed in the section below.
As reported in this subsection, SEM observations and XRD measurements demonstrated that polyFe coated the outside of the smaller planular particles, layered aggregates, and disordered agglomerates of LIIm to form polyFe/LIIm composites. The morphologies were varied for each polyFe/LIIm composite with varying polyFe/LIIm ratios. The morphologies of the polyFe/LIIm composite-3/0.1 were completely different compared to the other three composites. SEM studies demonstrated the polyFe/LIIm composite-3/5 was inhomogeneous. The polyFe/LIIm composite-3/1 exhibited the most disordered agglomerates among these composites.
3.2. UV-Vis Spectroscopy
Absorption peaks appeared in the UV-vis region for polyFe/LIIm composites and polyFe only (
Figure 3). The UV-vis spectra were normalized with the absorbance of peaks close to 335 nm in
Figure 3. The absorption peaks at ~335 nm are attributed to the π–π* transition of the ligand of the polyFe part. The UV-vis spectra showed that the absorption at ~600 nm was attributed to MLCT (d → π*) from Fe(II) ion to terpyridine moiety for polyFe/LIIm composites and polyFe. Interestingly, the MLCT absorptions (the intensities of normalized absorbance) were enhanced by combining with LIIm about polyFe/LIIm composites-3/1 and 3/0.1 (
Figure 3a,c). The absorbance of the MLCT absorption was comparable with that of the π–π* absorption for polyFe/LIIm composites-3/1, although the MLCT absorptions are significantly weaker than π–π* absorption in many cases for Fe-based complexes [
17] and polyFe–clay hybrids [
27]. The absorption close to 385 nm is attributed to the metal (Fe(II)) d–d transition of the polyFe part [
15,
27].
The absorption peaks of MLCT bands and d–d transition were redshifted for the polyFe/LIIm composites-3/0.1 (15 nm) and 3/1 (12 nm). These results suggest that the band gap energy decreased for the polyFe/LIIm composites-3/0.1 and 3/1. The absorptions attributed to the MLCT band were stronger, and absorptions caused by the d–d transition were weaker for the polyFe/LIIm composites-3/0.1 and 3/1 compared with polyFe only and other polyFe/LIIm composites-3/0.5 and 3/5. These changes suggest that transition probabilities are higher for the MLCT band and lower for the d–d transition about the polyFe/LIIm composites-3/0.1 and 3/1.
Different counter anions exert different influences on the bandgap energies of polyFe. Changes in these complexes, e.g., distortion in the octahedral structure, influence the bandgap energies [
13]. Redshifts for dyes and complexes immobilized on solid [
34] and dense solutions have been reported, and it has been demonstrated the redshifts are related to changes in their structures and the restriction of their mobilities, e.g., rotation and stabilization by the surrounding solids, such as solvation. We could speculate that the redshift of the MLCT band could be attributed to the exchange of the counter anion from the acetate anion to LIIm and, therefore, by stabilizing polyFe with LIIm. The absorption peaks attributed to the π–π* transition were not shifted. The results should demonstrate that the energy levels should not change for both the π and π* orbitals. When the energy level is stabilized for the d orbital but does not change for the π* orbital, the absorption should blueshift about the MLCT. Currently, the reason for the redshift in the MLCT absorption is being debated.
Interestingly the MLCT absorptions were enhanced and redshifted for polyFe/LIIm composites-3/1 and 0.1. The trend did not depend on the increasing amount of LIIm in composites. The complex trend could be related to the characteristic combining features with the most disordered morphologies for polyFe/LIIm composite-3/1. We will discuss this in detail below (
Section 3.5). We should note that the absorption of Ni(II) of LIIm should be overlapped on the absorption at ~385 nm. The broad absorption peaks appear at ~390 nm and 685 nm and are attributed to d–d transitions
3A
2g →
3T
1g (P) and
3A
2g →
3T
1g (F) of Ni(II) for LIIm [
30]. The UV-vis spectrum demonstrated a tailing for the MLCT band of the polyFe/LIIm composite-3/5 in a longer wavelength side (
Figure 3d). The tailing should be attributed to the overlap of absorption of the d–d transition
3A
2g →
3T
1g (F) of Ni(II). The absorption close to 385 nm is broad for the polyFe/LIIm composite-3/5. The broadening would have been attributed to the overlap with the broad absorption of
3A
2g →
3T
1g (P) of Ni(II). The overlap should have had more of an effect as the contents of LIIm increased in the polyFe/LIIm composites. Therefore, we recognized the overlaps for the polyFe/LIIm composite-3/5. Reflection absorption spectra were measured for the same samples. The overlaps were recognized for polyFe/LIIm composite-3/5 in the reflection absorption spectra (not shown).
PolyFe/LIIm composites exhibited a MLCT band at ~600 nm. Interestingly, the MLCT absorptions were enhanced and redshifted for polyFe/LIIm composites-3/1 and 3/0.1.
3.3. CV
In the cyclic voltammograms, a pair of redox peaks appeared at 0.86–0.89 V vs. Ag/Ag
+ for polyFe/LIIm composite cast films and 0.96 V for the reference polyFe cast film when applying potential to the positive side (scan rate: 50 mV/s) (
Figure 4). Peak currents in the vertical axis normalized the CV curves. The polyFe/LIIm composite and reference polyFe cast films turned transparent from blue-purple color around the peak when applying potential. The color change is attributed to the oxidation of Fe(II) ion to Fe(III) ion [
14,
15]. The peak potentials in CV are described to be oxidation potentials.
Color changes occurred at lower potentials for polyFe/LIIm composite cast films than the reference polyFe cast film. When the potential was applied to the opposite side (from 1.3 to 0 V), peaks appeared (
Figure 4), along with a color change to blue-purple from transparent. The changes in current and color were reversible, although
Figure 4 shows only the second cycle. Thus, polyFe/LIIm composite cast films, as well as polyFe, demonstrated reversible electrochromism.
CV measurements did not show any drastic change in half-wave potentials (E
1/2) for polyFe/LIIm composite cast films compared with the reference polyFe cast film. E
1/2 was 0.75–0.77 V for polyFe/LIIm composite cast films and 0.76 V for the reference polyFe cast film. The oxidation potential decreased, and the redox potential increased for polyFe/LIIm composite cast films compared with the reference polyFe cast film. Gaps between the oxidation and reduction potentials (ΔE) considerably decreased. ΔEs were 0.22–0.27 V for polyFe/LIIm composite cast films and 0.40 V for the reference polyFe cast film. Based on the reduction in ΔE, one can consider electron transfer at the interface between films and electrode becomes faster in the composites. ΔEs slightly increased for the previously reported polyFe–clay composites [
27,
29].
Scan-rate-dependent CV measurements were performed to better understand the redox mechanism (
Figure 5). The oxidation and reduction potentials shifted more as the scan speeds increased, and the peak currents increased for both polyFe/LIIm composite cast films and polyFe cast films (
Figure 5A). Both anodic and cathodic peak currents were linearly related to the square root of scan rates (
Figure 5B), and the scan rates were not proportional to the anodic and cathodic peak currents (
Figure S5, SI). Diffusion-controlled redox systems are shown by the linear dependency of peak currents on the square root of scan rates. The rate-determining step can be represented as the diffusion of counter anion(s).
With increase in the concentration of LIIm, the peak currents decreased to 0.75 (polyFe/LIIm composite-3/0.1)–0.57 (polyFe/LIIm composite-3/5) times that of polyFe, and slopes of the plots of peak currents vs. the square root of the scan rates decreased (
Figure 5B). It has been concluded based on Fick’s second law and the Nernst equation that the slope is proportional to diffusion coefficient in the diffusion-controlled system. The smaller slopes demonstrate that the counter anion(s) diffusion was slower by combining polyFe with LIIm. The slower diffusion of the counter anion (the acetate ion) would be primarily attributed to the presence of LIIm in the composites. We speculate the positive charge of polyFe could be compensated with the inorganic layer moiety with the negative charge in part and the acetate ion in polyFe/LIIm composites. Here, the counter anion (LIIm) would hardly diffuse.
The currents in the CV curves decreased but did not drastically decrease for the polyFe/LIIm composites compared with pure polyFe. Drastic decreases (~0.2 times) in the currents for polyFe intercalated in the interlayer space of saponite [
27] have been reported. It has been discussed in the literature [
27] that electron hopping rate could decrease by the layer space and by the less mobile counter anion, which should exist between layers in the SP–polyFe hybrid. The SEM and XRD experimental results demonstrated polyFe coats the LIIm aggregates in polyFe/LIIm composites. This would be a reason why the currents did not drastically decrease.
To conclude this subsection, polyFe/LIIm composites demonstrated a reversible pair of redox waves with accompanying reversible color changes from blue-purple color and transparent upon the application of potential with E1/2s of 0.75–0.77 V, which were comparable to that of polyFe, and ΔEs of 0.22–0.27 V, which were significantly smaller than that of polyFe. The reactions for electrochromism were diffusion-controlled redox systems with slower counter anion diffusion in polyFe/LIIm composites.
3.4. EC Properties
The blue-purple polyFe/LIIm composite-3/0.1 spin-coating film turned transparent upon applying a potential of 1.2 V vs. Ag/Ag
+ (insets of
Figure 6). Simultaneously, in situ spectro-electrochemical measurement demonstrated transmittance at 587 nm and became higher at 84.7% upon the applied potential of 1.2 V vs. Ag/Ag
+ from 8.7% before the applied potential (
Figure 6a,b). MLCT absorption disappeared upon the applying potential. The blue-purple color and MLCT absorption reappeared upon the applied potential being decreased to 0 V vs. Ag/Ag
+. Similarly, other polyFe/LIIm composites-3/0.5 and 1 spin-coating films demonstrated color changes and disappearance of MLCT absorption when potentials were applied. Another polyFe/LIIm composite-3/5 spin-coating film demonstrated a color change, but the polyFe/LIIm composite-3/5 spin-coating film was inhomogeneous upon applying the potential. There were blue-purple spots in the polyFe/LIIm composite-3/5 spin-coating film even upon using the potential.
For polyFe/LIIm composite-3/0.1, 0.5, and 1 spin-coating films, the transmittance contrasts (ΔT) between colored and bleached states were 76%, 70%, and 74%, respectively. The polyFe spin-coating film had a ΔT of 74%, and the ΔTs of polyFe/LIIm composites were comparable with polyFe film. They have previously reported that the polymer nanohybrids of polyFe have reduced ΔT. PolyFe was incorporated within a tube and on an outer surface of tubular hydroxylated halloysite [
29]. PolyFe covered the aggregation and agglomerations of LIIm in polyFe/LIIm composites. Therefore, large optical contrasts (ΔT) were maintained for polyFe/LIIm composites. Hu et al. previously reported hierarchically porous electrochromic film of nanocomposites comprising poly(3,4-ehylendioxythiophene) (PEDOT) coating on outer halloysites nanotubes with significantly higher ΔT of 59.3% than that of 15.3% for pure PEDOT [
23]. In this study, because we employed polyFe with a considerably high ΔT of 72% [
29] and polyFe coated the outer side of the aggregations and agglomerates of LIIm, polyFe/LIIm composites with high ΔT were achieved.
PolyFe/LIIm composites demonstrated significantly longer EC memory (
Figure 7). It took 785 s for polyFe/LIIm composite-3/1 film to recover 95% of the colored state in the open-circuit condition, although it took 236 s for the polyFe film. The logarithm of the transmittance (log T) exhibited linear decay vs. the elapsed time, i.e., the concentration of Fe
2+ ion linearly increased vs. time after oxidation (to re-reduction).
The retention times of the EC memory changed with polyFe/LIIm ratios of polyFe/LIIm composites, as listed in
Table 1. PolyFe/LIIm composites-3/0.1, 1, and 5 exhibited longer EC memories than polyFe in open and closed circuits. However, polyFe/LIIm composite-3/0.5 exhibited shorter EC memory than polyFe. The decays in the Fe(III) center were slower for the polyFe/LIIm composite-3/0.1 (closed-circuit), 3/1, and 3/5 films (both circuits) than the polyFe film. To reduce Fe
3+ centers, electron transfer on the interface between the films and electrodes is necessary, as is the diffusion of the counter anion(s) [
18]. The smaller ΔEs suggest that the electron transfers from the electrode to films were faster for polyFe/LIIm composite films than polyFe film. The smaller slopes (
Figure 5B) indicated the slower diffusion of counter anion(s) for polyFe/LIIm composite films. The shorter EC memory of polyFe/LIIm composite-3/0.5 would be attributed to the faster electron transfer on the electrode/composite film interface. This effect would influence the reduction rate more strongly than another effect by the slower diffusion of counter anion(s). The longer EC memories of other polyFe/LIIm composite films would be attributed to the slower diffusion of counter anion(s). Thus, the longer EC memories were achieved without the decrease in ΔT (larger than and/or equal to 70%) by combining polyFe with LIIm. However, retention times did not simply depend on the contents of LIIm. We will discuss this below.
3.5. The Improved Properties and Combining Features
The combination of polyFe with LIIm influenced the properties, i.e., the intensified and redshifted MLCT absorption, redshifted and weakened d–d transition absorption, and longer electrochromism memory while maintaining a large optical contrast (ΔT). However, the influence did not gradually enhance with increase in the LIIm contents. The stronger effects were detected for polyFe/LIIm composites-3/1 and 3/0.1.
The structural features were different for each polyFe/LIIm composite but did not gradually change with the LIIm contents. The experimental results suggested the particles of LIIm would be smaller in polyFe/LIIm composites-3/0.1 and 3/1 compared with polyFe/LIIm composites-3/0.5 and 3/5. The XRD measurements showed stronger scattering in the lowest angle region for polyFe/LIIm composites-3/0.1 and 3/1 (
Figure 2). The SEM observation demonstrated more disordered agglomerates for polyFe/LIIm composite-3/1 than polyFe/LIIm composites-3/0.5 and 3/5 (
Figure 1). The disordered agglomerates comprised smaller planular particles for polyFe/LIIm composite-3/1. The disordered agglomerates comprised layered aggregates and smaller planular particles for polyFe/LIIm composites-3/5 and 3/0.5. The surface area of LIIm contacting polyFe increased in the composites, when the smaller planular particles of LIIm were more dominant than the layered aggregates. We speculate that these differences in the combining features would be a reason for the stronger influence on polyFe/LIIm composites-3/1 and 3/0.1.
The larger effects in polyFe/LIIm composite-3/0.1 may evoke one possibility, where LIIm may be intercalated and exfoliated by polyFe in part. Indeed, this is consistent with the XRD data with relatively high scattering in the lowest angle region for polyFe/LIIm composites-3/0.1 and 3/1. LIIm is hardly swelled and exfoliated in MeOH; however, we speculate that LIIm would be possibly swelled and partly exfoliated in the mixture with a countless amount of MeOH and polyFe. We speculate that affinities between MeOH and Si–OH groups on the layer surface of LIIm and between the polyFe and imidazolyl group of LIIm could act as driving forces for swelling and exfoliation. There is an Si–OH group in the proposed structure for LIIm because of the steric hindrance of the imidazolyl group [
30], although there is no Si–OH group on the layer surface in the ideal structure of 2:1 phyllosilicates [
33,
34,
35,
36]. LIIm, partially intercalated and exfoliated, would strongly influence the MLCT and d–d transition bands, e.g., stabilizing the orbitals compared with LIIm comprising only the larger particles. LIIm would act as a counter anion of polyFe and stabilize the oxidized Fe(III) state, such as the electron-donating groups [
13]. The EC memory would be longer by stabilizing the oxidized Fe(III) state.
The EC memory properties were improved (
Figure 7) by combining with insulated LIIm. We consider the slower diffusion (
Figure 5B) of counter anion(s) in polyFe/LIIm composite films as the primary reason for improvement. Stabilizing the oxidized Fe(III) state by LIIm could be an additional reason for the long memory. When LIIm was the smaller planular particles and partly intercalated and exfoliated by polyFe, polyFe would be influenced more strongly by LIIm and the oxidized Fe(III) state was more stabilized.
The stronger combining effects on the properties were demonstrated for polyFe/LIIm composites-3/1 and 3/0.1 rather than effects gradually depending on the increasing ratios of LIIm. The combined features with smaller particles and higher surface area of LIIm contacting polyFe in polyFe/LIIm composites-3/1 and 3/0.1 would exhibit stronger effects.