Advances of Hydroxyapatite Hybrid Organic Composite Used as Drug or Protein Carriers for Biomedical Applications: A Review
Abstract
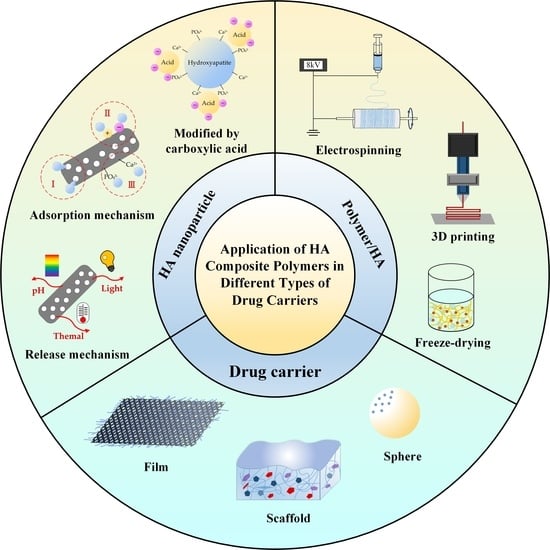
:1. Introduction
2. HA as a Template for Protein/Drug Carriers
2.1. Carrier of HANPs for Protein Adsorption
2.2. Effect of HA Structure on Drug Adsorption
3. Recent Strategies for Compounding Natural and Synthetic Polymers with HA
3.1. Electrospun Composites of HANPs/Organics
3.2. 3D Printing of Scaffolds for Tissue Engineering
3.3. Freeze Drying to Prepare Scaffolds
3.4. Other Techniques
4. Polymers–HA Composite as Carriers for Drug-Sustained Release
4.1. Membrane Form
4.2. Scaffold Form
4.3. Spherical Form
4.4. Coating
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorozhkin, S.V. A detailed history of calcium orthophosphates from 1770s till 1950. Mater. Sci. Eng. C 2013, 33, 3085–3110. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Ruiz-Hernández, E. Bioceramics: From bone regeneration to cancer nanomedicine. Adv. Mater. 2011, 23, 5177–5218. [Google Scholar] [CrossRef] [PubMed]
- Hilbrig, F.; Freitag, R. Isolation and purification of recombinant proteins, antibodies and plasmid DNA with hydroxyapatite chromatography. Biotechnol. J. 2012, 7, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.-J.; Lin, H.-L.; Haung, S.-M.; Liu, S.-M.; Chen, W.-C. Effect of ph on the in vitro biocompatibility of surfactant-assisted synthesis and hydrothermal precipitation of rod-shaped nano-hydroxyapatite. Polymers 2021, 13, 2994. [Google Scholar] [CrossRef]
- Chen, W.-C.; Cheng, I.-T.; Chang, K.-C.; Haung, S.-M.; Chen, J.-C.; Shih, C.-J. Heparin as a biomimetic template on nanoapatite rods with tunable aspect ratio: Synthesis and biocompatibility. J. Aust. Ceram. Soc. 2021, 1–10. [Google Scholar] [CrossRef]
- Chang, K.-C.; Chen, J.-C.; Cheng, I.-T.; Haung, S.-M.; Liu, S.-M.; Ko, C.-L.; Sun, Y.-S.; Shih, C.-J.; Chen, W.-C. Strength and biocompatibility of heparin-based calcium phosphate cement grafted with ferulic acid. Polymers 2021, 13, 2219. [Google Scholar] [CrossRef]
- Wang, J.; Gong, X.; Hai, J.; Li, T. Synthesis of silver–hydroxyapatite composite with improved antibacterial properties. Vacuum 2018, 152, 132–137. [Google Scholar] [CrossRef]
- Krithiga, G.; Sastry, T.P. Preparation and characterization of a novel bone graft composite containing bone ash and egg shell powder. Bull. Mater. Sci. 2011, 34, 177–181. [Google Scholar] [CrossRef]
- Haung, S.-M.; Chen, J.-C.; Chang, K.-C.; Ko, C.-L.; Lin, D.-J.; Chen, W.-C. Synthesis of nanorod apatites with templates at critical micelle concentrations and in vitro evaluation of cytotoxicity and antimicrobial activity. J. Asian Ceram. Soc. 2021, 1–12. [Google Scholar] [CrossRef]
- Wu, Y.R.; Chang, C.W.; Chang, K.C.; Lin, D.J.; Ko, C.L.; Wu, H.Y.; Chen, W.C. Effect of micro-/nano-hybrid hydroxyapatite rod reinforcement in composite resins on strength through thermal cycling. Polym. Compos. 2019, 40, 3703–3710. [Google Scholar] [CrossRef]
- Wu, Y.-R.; Chang, C.-W.; Chang, K.-C.; Ko, C.-L.; Wu, H.-Y.; Lin, J.-H.C.; Chen, W.-C. Characterization of hybrid light-cured resin composites reinforced by microspherical silanized DCPA/nanorod HA via thermal fatigue. J. Aust. Ceram. Soc. 2019, 55, 235–245. [Google Scholar] [CrossRef]
- Bin Mobarak, M.; Hossain, M.S.; Yeasmin, Z.; Mahmud, M.; Rahman, M.M.; Sultana, S.; Masum, S.M.; Ahmed, S. Probing the photocatalytic competency of hydroxyapatite synthesized by solid state and wet chemical precipitation method. J. Mol. Struct. 2022, 1252, 132142. [Google Scholar] [CrossRef]
- Gomez-Vazquez, O.M.; Correa-Piña, B.A.; Zubieta-Otero, L.F.; Castillo-Paz, A.M.; Londoño-Restrepo, S.M.; Rodriguez-García, M.E. Synthesis and characterization of bioinspired nano-hydroxyapatite by wet chemical precipitation. Ceram. Int. 2021, 47, 32775–32785. [Google Scholar] [CrossRef]
- Correa-Piña, B.A.; Gomez-Vazquez, O.M.; Londoño-Restrepo, S.M.; Zubieta-Otero, L.F.; Millan-Malo, B.M.; Rodriguez-García, M.E. Synthesis and characterization of nano-hydroxyapatite added with magnesium obtained by wet chemical precipitation. Prog. Nat. Sci. Mater. Int. 2021, 31, 575–582. [Google Scholar] [CrossRef]
- Markovic, M.; Fowler, B.O.; Tung, M.S. Preparation and comprehensive characterization of a calcium hydroxyapatite reference material. J. Res. Natl. Inst. Stand. Technol. 2004, 109, 553. [Google Scholar] [CrossRef]
- Jiao, Y.; Lu, Y.-P.; Xiao, G.-Y.; Xu, W.-H.; Zhu, R.-F. Preparation and characterization of hollow hydroxyapatite microspheres by the centrifugal spray drying method. Powder Technol. 2012, 217, 581–584. [Google Scholar] [CrossRef]
- Guo, X.; Yan, H.; Zhao, S.; Li, Z.; Li, Y.; Liang, X. Effect of calcining temperature on particle size of hydroxyapatite synthesized by solid-state reaction at room temperature. Adv. Powder Technol. 2013, 24, 1034–1038. [Google Scholar] [CrossRef]
- Jang, J.-H.; Oh, B.; Lee, E.-J. Crystalline hydroxyapatite/graphene oxide complex by low-temperature sol-gel synthesis and its characterization. Ceram. Int. 2021, 47, 27677–27684. [Google Scholar] [CrossRef]
- Saranya, S.; Prema Rani, M. Sol gel synthesis of Niobium influence on Hydroxyapatite: A view of invitro, structural, morphological and studies for Biomedical Applications. Mater. Today Proc 2021, 46, 1441–1450. [Google Scholar] [CrossRef]
- Abinaya Sindu, P.; Kolanthai, E.; Suganthi, R.V.; Thanigai Arul, K.; Manikandan, E.; Catalani, L.H.; Narayana Kalkura, S. Green synthesis of Si-incorporated hydroxyapatite using sodium metasilicate as silicon precursor and in vitro antibiotic release studies. J. Photochem. Photobiol. B Biol. 2017, 175, 163–172. [Google Scholar] [CrossRef]
- Sobhana, S.L.; Sundaraseelan, J.; Sekar, S.; Sastry, T.; Mandal, A. Gelatin–Chitosan composite capped gold nanoparticles: A matrix for the growth of hydroxyapatite. J. Nanopart. Res. 2009, 11, 333–340. [Google Scholar] [CrossRef]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Hou, Y.; Shavandi, A.; Carne, A.; Bekhit, A.A.; Ng, T.B.; Cheung, R.C.F.; Bekhit, A.E.-d.A. Marine shells: Potential opportunities for extraction of functional and health-promoting materials. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1047–1116. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Lee, D.K.; Park, J.Y.; Kim, M.R.; Jang, D.-J. Facile hydrothermal fabrication of hollow hexagonal hydroxyapatite prisms. CrystEngComm 2011, 13, 5455–5459. [Google Scholar] [CrossRef]
- Tsiourvas, D.; Tsetsekou, A.; Kammenou, M.I.; Boukos, N. Controlling the formation of hydroxyapatite nanorods with dendrimers. J. Am. Ceram. Soc. 2011, 94, 2023–2029. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhou, K.; Chen, C.; Jiang, M.; Zhang, Y.; Luo, H.; Zhang, D. Hollow and porous hydroxyapatite microspheres prepared with an O/W emulsion by spray freezing method. Mater. Sci. Eng. C 2016, 69, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Lett, J.A.; Sagadevan, S.; Latha, B.; Anandhi, S.; Ravichandran, S.; Murugesan, S. Development of porous guar gum-hydroxyapatite composite scaffolds via freeze-drying method. Mater. Today Proc 2021, 47, 1119–1122. [Google Scholar] [CrossRef]
- Sayed, M.; Mahmoud, E.M.; Bondioli, F.; Naga, S.M. Developing porous diopside/hydroxyapatite bio-composite scaffolds via a combination of freeze-drying and coating process. Ceram. Int. 2019, 45, 9025–9031. [Google Scholar] [CrossRef]
- Singh, G.; Singh, R.P.; Jolly, S.S. Customized hydroxyapatites for bone-tissue engineering and drug delivery applications: A review. J. Solgel. Sci. Technol. 2020, 94, 505–530. [Google Scholar] [CrossRef]
- Palierse, E.; Hélary, C.; Krafft, J.-M.; Génois, I.; Masse, S.; Laurent, G.; Alvarez Echazu, M.I.; Selmane, M.; Casale, S.; Valentin, L.; et al. Baicalein-modified hydroxyapatite nanoparticles and coatings with antibacterial and antioxidant properties. Mater. Sci. Eng. C 2021, 118, 111537. [Google Scholar] [CrossRef]
- Zhang, R.; Metoki, N.; Sharabani-Yosef, O.; Zhu, H.; Eliaz, N. Hydroxyapatite/Mesoporous Graphene/Single-Walled Carbon Nanotubes Freestanding Flexible Hybrid Membranes for Regenerative Medicine. Adv. Funct. Mater. 2016, 26, 7965–7974. [Google Scholar] [CrossRef]
- Qi, C.; Lin, J.; Fu, L.-H.; Huang, P. Calcium-based biomaterials for diagnosis, treatment, and theranostics. Chem. Soc. Rev. 2018, 47, 357–403. [Google Scholar] [CrossRef]
- Mondal, S.; Hoang, G.; Manivasagan, P.; Kim, H.; Oh, J. Nanostructured hollow hydroxyapatite fabrication by carbon templating for enhanced drug delivery and biomedical applications. Ceram. Int. 2019, 45, 17081–17093. [Google Scholar] [CrossRef]
- Benedini, L.; Placente, D.; Ruso, J.; Messina, P. Adsorption/desorption study of antibiotic and anti-inflammatory drugs onto bioactive hydroxyapatite nano-rods. Mater. Sci. Eng. C 2019, 99, 180–190. [Google Scholar] [CrossRef]
- Cai, A.Y.; Zhu, Y.J.; Qi, C. Biodegradable inorganic nanostructured biomaterials for drug delivery. Adv. Mater. Interfaces 2020, 7, 2000819. [Google Scholar] [CrossRef]
- Placente, D.; Benedini, L.A.; Baldini, M.; Laiuppa, J.A.; Santillán, G.E.; Messina, P.V. Multi-drug delivery system based on lipid membrane mimetic coated nano-hydroxyapatite formulations. Int. J. Pharm. 2018, 548, 559–570. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, Y.; Hong, X.; Liu, Z.; Yuan, W. Porous microsphere and its applications. Int. J. Nanomed. 2013, 8, 1111. [Google Scholar] [CrossRef]
- Lee, W.H.; Loo, C.Y.; Zavgorodniy, A.V.; Rohanizadeh, R. High protein adsorptive capacity of amino acid-functionalized hydroxyapatite. J. Biomed. Mater. Res. A 2013, 101, 873–883. [Google Scholar] [CrossRef]
- Almora-Barrios, N.; de Leeuw, N.H. Modelling the interaction of a Hyp-Pro-Gly peptide with hydroxyapatite surfaces in aqueous environment. CrystEngComm 2010, 12, 960–967. [Google Scholar] [CrossRef]
- D’Elia, N.L.; Gravina, N.; Ruso, J.M.; Marco-Brown, J.L.; Sieben, J.M.; Messina, P.V. Albumin-mediated deposition of bone-like apatite onto nano-sized surfaces: Effect of surface reactivity and interfacial hydration. J. Colloid Interface Sci. 2017, 494, 345–354. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, Y.; Wang, C.; Sun, R.; Tang, Y. Effects of physico-chemical properties of ions-doped hydroxyapatite on adsorption and release performance of doxorubicin as a model anticancer drug. Mater. Chem. Phys. 2022, 276, 125440. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, D.; Sun, R.; Shang, S.; Wang, H.; Yang, Y.; Wang, L.; Liu, X.; Sun, T.; Chen, K. Adsorption behavior of drugs on hydroxyapatite with different morphologies: A combined experimental and molecular dynamics simulation study. Ceram. Int. 2019, 45, 19522–19527. [Google Scholar] [CrossRef]
- Yu, Y.-D.; Zhu, Y.-J.; Qi, C.; Jiang, Y.-Y.; Li, H.; Wu, J. Hydroxyapatite nanorod-assembled porous hollow polyhedra as drug/protein carriers. J. Colloid Interface Sci. 2017, 496, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Chen, J.; Liu, K.; Xing, H.; Song, C. Recent advances in biomedical engineering of nano-hydroxyapatite including dentistry, cancer treatment and bone repair. Compos. B. Eng. 2021, 215, 108790. [Google Scholar] [CrossRef]
- Anita Lett, J.; Sagadevan, S.; Fatimah, I.; Hoque, M.E.; Lokanathan, Y.; Léonard, E.; Alshahateet, S.F.; Schirhagl, R.; Oh, W.C. Recent advances in natural polymer-based hydroxyapatite scaffolds: Properties and applications. Eur. Polym. J. 2021, 148, 110360. [Google Scholar] [CrossRef]
- Swetha, M.; Sahithi, K.; Moorthi, A.; Srinivasan, N.; Ramasamy, K.; Selvamurugan, N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int. J. Biol. Macromol. 2010, 47, 1–4. [Google Scholar] [CrossRef]
- Kadu, K.; Kowshik, M.; Roy Ramanan, S. Does the nanoparticle morphology influence interaction with protein: A case study with hydroxyapatite nanoparticles. Mater. Today Commun. 2021, 26, 102172. [Google Scholar] [CrossRef]
- Zhang, N.; Gao, T.; Wang, Y.; Wang, Z.; Zhang, P.; Liu, J. Environmental pH-controlled loading and release of protein on mesoporous hydroxyapatite nanoparticles for bone tissue engineering. Mater. Sci. Eng. C 2015, 46, 158–165. [Google Scholar] [CrossRef]
- He, Q.; Pan, L.; Wang, Y.; Meldrum, F.C. Bioinspired synthesis of large-pore, mesoporous hydroxyapatite nanocrystals for the controlled release of large pharmaceutics. Cryst. Growth Des. 2015, 15, 723–731. [Google Scholar] [CrossRef]
- Zhang, C.; Shan, S.; Hu, T.; Wang, G.; Zhi, Y.; Su, H.; Jiang, L.; Ni, Y. Recent progress on biomedical applications of functionalized hollow hydroxyapatite microspheres. Ceram. Int. 2021, 47, 13552–13571. [Google Scholar] [CrossRef]
- Chen, R.; Shi, J.; Zhu, B.; Zhang, L.; Cao, S. Mesoporous hollow hydroxyapatite capped with smart polymer for multi-stimuli remotely controlled drug delivery. Microporous Mesoporous Mater. 2020, 306, 110447. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Liu, C.-H.; Liang, Y.-H.; Lin, F.-H.; Wu, K.C.-W. Hollow mesoporous hydroxyapatite nanoparticles (hmHANPs) with enhanced drug loading and pH-responsive release properties for intracellular drug delivery. J. Mater. Chem. B 2013, 1, 2447–2450. [Google Scholar] [CrossRef]
- Bastakoti, B.P.; Hsu, Y.C.; Liao, S.H.; Wu, K.C.W.; Inoue, M.; Yusa, S.i.; Nakashima, K.; Yamauchi, Y. Inorganic–organic hybrid nanoparticles with biocompatible calcium phosphate thin shells for fluorescence enhancement. Chem. Asian J. 2013, 8, 1301–1305. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-H.; Liu, C.-H.; Liao, S.-H.; Lin, Y.-Y.; Tang, H.-W.; Liu, S.-Y.; Lai, I.-R.; Wu, K.C.-W. Cosynthesis of cargo-loaded hydroxyapatite/alginate core–shell nanoparticles (HAP@ Alg) as pH-responsive nanovehicles by a pre-gel method. ACS Appl. Mater. Interfaces 2012, 4, 6720–6727. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.U.; Ihsan, A.; Sarwar, Y.; Bajwa, S.Z.; Bano, K.; Tehseen, B.; Zeb, N.; Hussain, I.; Ansari, M.T.; Saeed, M.; et al. Hollow mesoporous hydroxyapatite nanostructures; smart nanocarriers with high drug loading and controlled releasing features. Int. J. Pharm. 2018, 544, 112–120. [Google Scholar] [CrossRef]
- Safi, S.; Karimzadeh, F.; Labbaf, S. Mesoporous and hollow hydroxyapatite nanostructured particles as a drug delivery vehicle for the local release of ibuprofen. Mater. Sci. Eng. C 2018, 92, 712–719. [Google Scholar] [CrossRef]
- Ishihara, S.; Matsumoto, T.; Onoki, T.; Uddin, M.H.; Sohmura, T.; Nakahira, A. Regulation of the protein-loading capacity of hydroxyapatite by mercaptosuccinic acid modification. Acta Biomater. 2010, 6, 830–835. [Google Scholar] [CrossRef]
- Lee, W.-H.; Loo, C.-Y.; Rohanizadeh, R. Functionalizing the surface of hydroxyapatite drug carrier with carboxylic acid groups to modulate the loading and release of curcumin nanoparticles. Mater. Sci. Eng. C 2019, 99, 929–939. [Google Scholar] [CrossRef]
- Chang, K.-C.; Chen, W.-C.; Haung, S.-M.; Liu, S.-M.; Lin, C.-L. Effects of hinokitiol and dicalcium phosphate on the osteoconduction and antibacterial activity of gelatin-hyaluronic acid crosslinked hydrogel membrane in vitro. Pharmaceuticals 2021, 14, 802. [Google Scholar] [CrossRef]
- Chang, K.-C.; Lin, D.-J.; Wu, Y.-R.; Chang, C.-W.; Chen, C.-H.; Ko, C.-L.; Chen, W.-C. Characterization of genipin-crosslinked gelatin/hyaluronic acid-based hydrogel membranes and loaded with hinokitiol: In vitro evaluation of antibacterial activity and biocompatibility. Mater. Sci. Eng. C 2019, 105, 110074. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-C.; Chen, W.-C.; Chen, C.-H.; Ko, C.-L.; Liu, S.-M.; Chen, J.-C. Chemical cross-linking on gelatin-hyaluronan loaded with hinokitiol for the preparation of guided tissue regeneration hydrogel membranes with antibacterial and biocompatible properties. Mater. Sci. Eng. C 2021, 119, 111576. [Google Scholar] [CrossRef] [PubMed]
- Haung, S.-M.; Lin, Y.-T.; Liu, S.-M.; Chen, J.-C.; Chen, W.-C. In Vitro Evaluation of a Composite Gelatin–Hyaluronic Acid–Alginate Porous Scaffold with Different Pore Distributions for Cartilage Regeneration. Gels 2021, 7, 165. [Google Scholar] [CrossRef] [PubMed]
- Sonseca, A.; Peponi, L.; Sahuquillo, O.; Kenny, J.M.; Giménez, E. Electrospinning of biodegradable polylactide/hydroxyapatite nanofibers: Study on the morphology, crystallinity structure and thermal stability. Polym. Degrad. Stab. 2012, 97, 2052–2059. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, F.V.; Otoni, C.G.; Lopes, J.H.; de Souza, L.P.; Mei, L.H.I.; Lona, L.M.F.; Lozano, K.; Lobo, A.O.; Mattoso, L.H.C. Ultrathin polymer fibers hybridized with bioactive ceramics: A review on fundamental pathways of electrospinning towards bone regeneration. Mater. Sci. Eng. C 2021, 123, 111853. [Google Scholar] [CrossRef]
- Deng, L.; Li, Y.; Zhang, A.; Zhang, H. Nano-hydroxyapatite incorporated gelatin/zein nanofibrous membranes: Fabrication, characterization and copper adsorption. Int. J. Biol. Macromol. 2020, 154, 1478–1489. [Google Scholar] [CrossRef]
- Xie, X.; Chen, Y.; Wang, X.; Xu, X.; Shen, Y.; Khan, A.u.R.; Aldalbahi, A.; Fetz, A.E.; Bowlin, G.L.; El-Newehy, M.; et al. Electrospinning nanofiber scaffolds for soft and hard tissue regeneration. J. Mater. Sci. Technol. 2020, 59, 243–261. [Google Scholar] [CrossRef]
- Sebastian, T.; Preisker, T.R.; Gorjan, L.; Graule, T.; Aneziris, C.G.; Clemens, F.J. Synthesis of hydroxyapatite fibers using electrospinning: A study of phase evolution based on polymer matrix. J. Eur. Ceram. Soc. 2020, 40, 2489–2496. [Google Scholar] [CrossRef]
- Watcharajittanont, N.; Tabrizian, M.; Putson, C.; Pripatnanont, P.; Meesane, J. Osseointegrated membranes based on electro-spun TiO2/hydroxyapatite/polyurethane for oral maxillofacial surgery. Mater. Sci. Eng. C 2020, 108, 110479. [Google Scholar] [CrossRef]
- Shirzaei Sani, I.; Rezaei, M.; Baradar Khoshfetrat, A.; Razzaghi, D. Preparation and characterization of polycaprolactone/chitosan-g-polycaprolactone/hydroxyapatite electrospun nanocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2021, 182, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cai, N.; Chan, V.; Zeng, H.; Shi, H.; Xue, Y.; Yu, F. Antimicrobial hydroxyapatite reinforced-polyelectrolyte complex nanofibers with long-term controlled release activity for potential wound dressing application. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126722. [Google Scholar] [CrossRef]
- Chuan, D.; Fan, R.; Wang, Y.; Ren, Y.; Wang, C.; Du, Y.; Zhou, L.; Yu, J.; Gu, Y.; Chen, H.; et al. Stereocomplex poly(lactic acid)-based composite nanofiber membranes with highly dispersed hydroxyapatite for potential bone tissue engineering. Compos. Sci. Technol. 2020, 192, 108107. [Google Scholar] [CrossRef]
- Chen, P.; Liu, L.; Pan, J.; Mei, J.; Li, C.; Zheng, Y. Biomimetic composite scaffold of hydroxyapatite/gelatin-chitosan core-shell nanofibers for bone tissue engineering. Mater. Sci. Eng. C 2019, 97, 325–335. [Google Scholar] [CrossRef]
- Marew, T.; Birhanu, G. Three dimensional printed nanostructure biomaterials for bone tissue engineering. Regen. Ther. 2021, 18, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, S.; Vijayavenkataraman, S. Design of 3D printed scaffolds for bone tissue engineering: A review. Bioprinting 2021, 24, e00167. [Google Scholar] [CrossRef]
- Zhang, H.; Jiao, C.; He, Z.; Ge, M.; Tian, Z.; Wang, C.; Wei, Z.; Shen, L.; Liang, H. Fabrication and properties of 3D printed zirconia scaffold coated with calcium silicate/hydroxyapatite. Ceram. Int. 2021, 47, 27032–27041. [Google Scholar] [CrossRef]
- Iglesias-Mejuto, A.; García-González, C.A. 3D-printed alginate-hydroxyapatite aerogel scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2021, 131, 112525. [Google Scholar] [CrossRef]
- Cestari, F.; Petretta, M.; Yang, Y.; Motta, A.; Grigolo, B.; Sglavo, V.M. 3D printing of PCL/nano-hydroxyapatite scaffolds derived from biogenic sources for bone tissue engineering. Sustain. Mater. Technol. 2021, 29, e00318. [Google Scholar] [CrossRef]
- Wei, J.; Yan, Y.; Gao, J.; Li, Y.; Wang, R.; Wang, J.; Zou, Q.; Zuo, Y.; Zhu, M.; Li, J. 3D-printed hydroxyapatite microspheres reinforced PLGA scaffolds for bone regeneration. Mater. Sci. Eng. C 2021, 112618. [Google Scholar] [CrossRef]
- Chen, S.; Shi, Y.; Zhang, X.; Ma, J. 3D printed hydroxyapatite composite scaffolds with enhanced mechanical properties. Ceram. Int. 2019, 45, 10991–10996. [Google Scholar] [CrossRef]
- Yeo, T.; Ko, Y.-G.; Kim, E.J.; Kwon, O.K.; Chung, H.Y.; Kwon, O.H. Promoting bone regeneration by 3D-printed poly(glycolic acid)/hydroxyapatite composite scaffolds. J. Ind. Eng. Chem. 2021, 94, 343–351. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, H.; Lee, J. Various preparation methods of highly porous hydroxyapatite/polymer nanoscale biocomposites for bone regeneration. Acta Biomater. 2011, 7, 3813–3828. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Wu, W.; Wei, Y.; Ren, L.; Lin, S.; Wu, J. Biomimetic gelatin/chitosan/polyvinyl alcohol/nano-hydroxyapatite scaffolds for bone tissue engineering. Mater. Des. 2021, 207, 109865. [Google Scholar] [CrossRef]
- Xing, F.; Chi, Z.; Yang, R.; Xu, D.; Cui, J.; Huang, Y.; Zhou, C.; Liu, C. Chitin-hydroxyapatite-collagen composite scaffolds for bone regeneration. Int. J. Biol. Macromol. 2021, 184, 170–180. [Google Scholar] [CrossRef]
- Pottathara, Y.B.; Vuherer, T.; Maver, U.; Kokol, V. Morphological, mechanical, and in-vitro bioactivity of gelatine/collagen/hydroxyapatite based scaffolds prepared by unidirectional freeze-casting. Polym. Test. 2021, 102, 107308. [Google Scholar] [CrossRef]
- Kane, R.J.; Roeder, R.K. Effects of hydroxyapatite reinforcement on the architecture and mechanical properties of freeze-dried collagen scaffolds. J. Mech. Behav. Biomed. Mater. 2012, 7, 41–49. [Google Scholar] [CrossRef]
- Brahimi, S.; Ressler, A.; Boumchedda, K.; Hamidouche, M.; Kenzour, A.; Djafar, R.; Antunović, M.; Bauer, L.; Hvizdoš, P.; Ivanković, H. Preparation and characterization of biocomposites based on chitosan and biomimetic hydroxyapatite derived from natural phosphate rocks. Mater. Chem. Phys. 2022, 276, 125421. [Google Scholar] [CrossRef]
- Feroz, S.; Dias, G. Hydroxypropylmethyl cellulose (HPMC) crosslinked keratin/hydroxyapatite (HA) scaffold fabrication, characterization and in vitro biocompatibility assessment as a bone graft for alveolar bone regeneration. Heliyon 2021, 7, e08294. [Google Scholar] [CrossRef]
- El-Meliegy, E.; Abu-Elsaad, N.; El-Kady, A.M.; Ibrahim, M.A. Improvement of physico-chemical properties of dextran-chitosan composite scaffolds by addition of nano-hydroxyapatite. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Nabavinia, M.; Khoshfetrat, A.B.; Naderi-Meshkin, H. Nano-hydroxyapatite-alginate-gelatin microcapsule as a potential osteogenic building block for modular bone tissue engineering. Mater. Sci. Eng. C 2019, 97, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.E.J.; Volnistem, E.A.; Dias, G.S.; Cótica, L.F.; Santos, I.A.; Fiorentin, E.R.; de Oliveira, M.A.; Witchemichen, D.H.; Freitas, V.F.; Bonadio, T.G.M. Polyvinylidene fluoride—Hydroxyapatite 0–3 biocomposite filaments processed by twin-screw extrusion. J. Mech. Behav. Biomed. Mater. 2022, 125, 104891. [Google Scholar] [CrossRef] [PubMed]
- Wenzhi, S.; Dezhou, W.; Min, G.; Chunyu, H.; Lanlan, Z.; Peibiao, Z. Assessment of nano-hydroxyapatite and poly (lactide-co-glycolide) nanocomposite microspheres fabricated by novel airflow shearing technique for in vivo bone repair. Mater. Sci. Eng. C 2021, 128, 112299. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, E.M.; Sayed, M.; El-Kady, A.M.; Elsayed, H.; Naga, S.M. In vitro and in vivo study of naturally derived alginate/hydroxyapatite bio composite scaffolds. Int. J. Biol. Macromol. 2020, 165, 1346–1360. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Wang, A. In situ generation of sodium alginate/hydroxyapatite nanocomposite beads as drug-controlled release matrices. Acta Biomater. 2010, 6, 445–454. [Google Scholar] [CrossRef]
- Eskitoros-Togay, Ş.M.; Bulbul, Y.E.; Dilsiz, N. Combination of nano-hydroxyapatite and curcumin in a biopolymer blend matrix: Characteristics and drug release performance of fibrous composite material systems. Int. J. Pharm. 2020, 590, 119933. [Google Scholar] [CrossRef]
- Türe, H. Characterization of hydroxyapatite-containing alginate–gelatin composite films as a potential wound dressing. Int. J. Biol. Macromol. 2019, 123, 878–888. [Google Scholar] [CrossRef]
- Prakash, J.; Kumar, T.S.; Venkataprasanna, K.S.; Niranjan, R.; Kaushik, M.; Samal, D.B.; Venkatasubbu, G.D. PVA/alginate/hydroxyapatite films for controlled release of amoxicillin for the treatment of periodontal defects. Appl. Surf. Sci. 2019, 495, 143543. [Google Scholar] [CrossRef]
- Ramírez-Agudelo, R.; Scheuermann, K.; Gala-García, A.; Monteiro, A.P.F.; Pinzón-García, A.D.; Cortés, M.E.; Sinisterra, R.D. Hybrid nanofibers based on poly-caprolactone/gelatin/hydroxyapatite nanoparticles-loaded Doxycycline: Effective anti-tumoral and antibacterial activity. Mater. Sci. Eng. C 2018, 83, 25–34. [Google Scholar] [CrossRef]
- Baldino, L.; Aragón, J.; Mendoza, G.; Irusta, S.; Cardea, S.; Reverchon, E. Production, characterization and testing of antibacterial PVA membranes loaded with HA-Ag3PO4 nanoparticles, produced by SC-CO2 phase inversion. J. Chem. Technol. Biotechnol. 2019, 94, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-W.; Knowles, J.C.; Kim, H.-E. Hydroxyapatite/poly(ε-caprolactone) composite coatings on hydroxyapatite porous bone scaffold for drug delivery. Biomaterials 2004, 25, 1279–1287. [Google Scholar] [CrossRef]
- Zhang, B.; Nguyen, A.K.; Narayan, R.J.; Huang, J. Direct ink writing of vancomycin-loaded polycaprolactone/polyethylene oxide/hydroxyapatite 3D scaffolds. J. Am. Ceram. Soc. 2021. [Google Scholar] [CrossRef]
- Martínez-Vázquez, F.J.; Cabañas, M.V.; Paris, J.L.; Lozano, D.; Vallet-Regí, M. Fabrication of novel Si-doped hydroxyapatite/gelatine scaffolds by rapid prototyping for drug delivery and bone regeneration. Acta Biomater. 2015, 15, 200–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Zou, S.; Chen, W.; Tong, Z.; Wang, C. Mineralization and drug release of hydroxyapatite/poly(l-lactic acid) nanocomposite scaffolds prepared by Pickering emulsion templating. Colloids Surf. B 2014, 122, 559–565. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Wang, J.; Qu, Y.; Liu, G. In vivo drug release and antibacterial properties of vancomycin loaded hydroxyapatite/chitosan composite. Drug Deliv. 2012, 19, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Wu, J.; Li, F.; Huang, Z.; Pi, Y.; Miao, G.; Ren, W.; Liu, T.; Jiang, Q.; Guo, L. Drug-loading three-dimensional scaffolds based on hydroxyapatite-sodium alginate for bone regeneration. J. Biomed. Mater. Res. A 2021, 109, 219–231. [Google Scholar] [CrossRef]
- Bi, Y.-g.; Lin, Z.-t.; Deng, S.-t. Fabrication and characterization of hydroxyapatite/sodium alginate/chitosan composite microspheres for drug delivery and bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Teng, S.-H.; Niu, N.; Wang, P. Biomimetic synthesis of novel polyvinyl alcohol/hydroxyapatite composite microspheres for biomedical applications. Mater. Res. Express 2018, 5, 115401. [Google Scholar] [CrossRef]
- He, J.; Hu, X.; Cao, J.; Zhang, Y.; Xiao, J.; Peng, l.; Chen, D.; Xiong, C.; Zhang, L. Chitosan-coated hydroxyapatite and drug-loaded polytrimethylene carbonate/polylactic acid scaffold for enhancing bone regeneration. Carbohydr. Polym. 2021, 253, 117198. [Google Scholar] [CrossRef]
- Calasans-Maia, M.D.; Junior, C.A.B.B.; Soriano-Souza, C.A.; Alves, A.T.N.N.; de Pinheiro Uzeda, M.J.; Martinez-Zelaya, V.R.; Mavropoulos, E.; Leão, M.H.R.; de Santana, R.B.; Granjeiro, J.M. Microspheres of alginate encapsulated minocycline-loaded nanocrystalline carbonated hydroxyapatite: Therapeutic potential and effects on bone regeneration. Int. J. Nanomed. 2019, 14, 4559. [Google Scholar] [CrossRef] [Green Version]
- Padmanabhan, V.P.; Kulandaivelu, R.; Nellaiappan, S.N.T.S. New core-shell hydroxyapatite/Gum-Acacia nanocomposites for drug delivery and tissue engineering applications. Mater. Sci. Eng. C 2018, 92, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, A.; Venkatasubbu, G.D. Sustained release of amoxicillin from hydroxyapatite nanocomposite for bone infections. Prog. Biomater. 2018, 7, 289–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, S.; Vu, A.A.; Emshadi, K.; Bandyopadhyay, A. Effects of polycaprolactone on alendronate drug release from Mg-doped hydroxyapatite coating on titanium. Mater. Sci. Eng. C 2018, 88, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Mu, Z.; Yu, Y.; Zhang, L.; Hu, J. Polyethyleneimine-stabilized hydroxyapatite nanoparticles modified with hyaluronic acid for targeted drug delivery. RSC Adv. 2016, 6, 101790–101799. [Google Scholar] [CrossRef]






| HANP Structures | Proteins/Drug | Highlights and Potential Clinical Applications | Ref. |
|---|---|---|---|
| Solid (non-porous) hydroxyapatite nanoparticles (HANPs) | Pepsin A | Comparing the effects of different types of HA modified with cetyl pyridine chloride on the interaction with pepsin A, HANPs have higher enzymatic activity (18.45%) than microscale. HANPs with surface modification can improve their use in biomedical applications potential. | [49] |
| Mesoporous hydroxyapatite nanoparticles (M-HANPs) | Bovine serum albumin (BSA) | The adsorption capacity of M-HANPs in acidic environment (pH 4.7) was higher than that of micro-HA particles. In alkaline environments (pH 8.4), they have smaller bursts and flatter release profiles, which can be used for targeted drug delivery and bone therapy. | [50] |
| Mesoporous hydroxyapatite rod-like nanocrystals | Fetuin from serum protein | Fetuin has the ability to inhibit the growth of M-HA nanocrystals to form dumbbell shaped, mesoporous structure, and large surface area. M-HAs of rod-like crystal size (235–515 nm) with inner mesopores (21–31 nm) can load more drugs and sustained-release drugs, which is beneficial to the field of drug delivery and sustained-release as drug delivery vehicles. | [51] |
| Hollow mesoporous hydroxyapatite nanoparticles | Doxorubicin (DOX) | The hollow mesoporous structure of M-HANPs has high biocompatibility and good drug loading capacity, the drug loading rate is increased from 17.9% to 93.7%, and has excellent drug nanocarrier performance as carriers of large pharmaceutics. | [54] |
| Solid and mesoporous hydroxyapatite nanoparticles | Ciprofloxacin | Compared with solid HANPs, M-HANPs have higher specific surface area and high drug loading, and have greater application potential in the field of drug delivery. Therefore, M-HANPs can potentially be used in smart drug delivery systems. | [57] |
| Functionalization of hydroxyapatite nanoparticles | curcumin nanoparticles | Carboxylic acid surface modification of HANPs can enhance the adsorption of curcumin and improve its drug availability. Curcumin-modified HANPs have better anticancer activity and have good potential in the field of medical regeneration. | [60] |
| Biomolecules with Different Types of Appearance | Drug | Highlights and Potential Clinical Applications | Ref. |
|---|---|---|---|
| TCH/HANPs/CG core–shell nanofibers | Tetracycline hydrochloride (TCH) | The composite nanofibers have long-lasting antibacterial function, good biocompatibility, and high mechanical strength, and are suitable for wound dressings and drug delivery systems. | [72] |
| HANPs/PLGA microspheres | − | The diameter of the composite microspheres is about 250 μm. When the content of HANPs was 20% and 40%, respectively, it could promote the mineralization and osteogenic differentiation of MC3T3-E1 cells, and had good clinical application potential in bone tissue engineering and bone implantation. | [93] |
| HANPs-containing alginate–gelatin composite films | Tetracycline hydrochloride (TCH) | The addition of HANPs will make the surface of the composite film rougher and effectively improve the thermal stability. In addition, it can reduce the initial burst release of the drug. The polymer-HA composite film can be used not only for biomedical applications, but also for food packaging. | [97] |
| Polycaprolactone/ polyethylene oxide/ hydroxyapatite 3D scaffolds | Vancomycin (VCM) | The composite scaffold with HA content of 65% had the best wettability and mechanical properties, but adding too much HA would affect the mechanical properties of the polymer-HA composite. The drug release showed an initial burst, and the 3D scaffold with antibacterial activity was suitable for bone tissue engineering applications. | [102] |
| A chitosan (CS)-coated polytrimethylene carbonate (PTMC)/polylactic acid (PLLA)/oleic acid-modified hydroxyapatite (OA-HA)/vancomycin hydrochloride (VH) microsphere scaffold | vancomycin hydrochloride (VH) | Two active molecules, OA-HA and VH, can be released through the pores. In addition to facilitating osteoblast adhesion, CS coating can also control the release behavior of the OA-HA to stimulate the proliferation of osteoblasts, which is expected to be used in bone tissue engineering. | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-M.; Liu, S.-M.; Ko, C.-L.; Chen, W.-C. Advances of Hydroxyapatite Hybrid Organic Composite Used as Drug or Protein Carriers for Biomedical Applications: A Review. Polymers 2022, 14, 976. https://doi.org/10.3390/polym14050976
Huang S-M, Liu S-M, Ko C-L, Chen W-C. Advances of Hydroxyapatite Hybrid Organic Composite Used as Drug or Protein Carriers for Biomedical Applications: A Review. Polymers. 2022; 14(5):976. https://doi.org/10.3390/polym14050976
Chicago/Turabian StyleHuang, Ssu-Meng, Shih-Ming Liu, Chia-Ling Ko, and Wen-Cheng Chen. 2022. "Advances of Hydroxyapatite Hybrid Organic Composite Used as Drug or Protein Carriers for Biomedical Applications: A Review" Polymers 14, no. 5: 976. https://doi.org/10.3390/polym14050976
APA StyleHuang, S. -M., Liu, S. -M., Ko, C. -L., & Chen, W. -C. (2022). Advances of Hydroxyapatite Hybrid Organic Composite Used as Drug or Protein Carriers for Biomedical Applications: A Review. Polymers, 14(5), 976. https://doi.org/10.3390/polym14050976