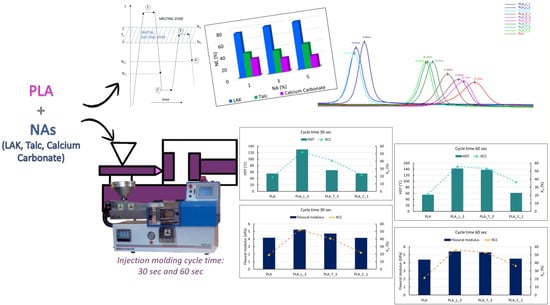
Improvement of the PLA Crystallinity and Heat Distortion Temperature Optimizing the Content of Nucleating Agents and the Injection Molding Cycle Time
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- Poly(lactic) acid (PLA), trade name PLA 3100 HP, purchased from Natureworks LLC (Minneapolis, MN, USA). It is commercial-grade PLA (~0.3% of D content) derived from natural resources and designed for injection molding applications (density: 1.24 g/cm3; melt flow index (MFI) (210 °C/2.16 kg): 24 g/10 min, Mw = 148,250 g/mol).
- Potassium salt of 3,5-bis(methoxycarbonyl)benzenesulfonate, trade name LAK-301, produced by Takemoto Oil & Fat (Minatomachi, Japan), is an aromatic sulphonate derivative nucleating agent. It appears as a whitish powder, with a specific gravity 1.668 g/cm3 and particle size around 10 μm.
- Jetfine® 0.7 CA talc provided by IMERYS (Paris, France). It is an ultrafine-grind lamellar talc able to improve the nucleation in crystalline polymers. It appears as a very white powder, with specific gravity of 2.78 g/cm3 and medium particle size of 0.7 μm.
- Socal® 312 calcium carbonate was also provided by IMERYS (Paris, France). It is an ultrafine, white and odorless, organic, surface coated and precipitated calcium carbonate. It is a powder with unique crystal size and shape (density: 2.71 g/cm3; particle diameter: 0.05–0.09 μm; surface area: 18 m2/g; coating content: 24–33 g/kg).
2.2. PLA Formulation Extrusion and Injection Molding
2.3. Thermal Characterizations
2.3.1. Non-Isothermal Crystallization
2.3.2. Isothermal Crystallization
2.3.3. Final Thermal Properties and Crystallinity of the Injection Molded Specimens
2.3.4. Self-Nucleation and Nucleating Efficiency Evaluation
- Erasing of the sample thermal history: in this first step, PLA was rapidly heated at 200 °C/min to 210 °C and held at this temperature for 5 min to erase its thermal history.
- Creation of the standard state (Tc1): this standard state is obtained by cooling the sample from point 1 at 10 °C/min to a temperature below its cold crystallization temperature. For this step, a temperature equal to 65 °C was chosen. During this step, the crystallization takes place at the lower limit of the crystallization range (Tc1) depending on the molecular polymer characteristics.
- Partial melting self-nucleation: this is the fundamental step for self-nucleation and it was obtained by heating the sample at 10 °C/min to selected temperatures, Ts, ranging from Ts1 = 164 °C to Ts2 = 171 °C; then it followed an isothermal step of 5 min. The Ts is located in the temperature range illustrated in Figure 1b where the formation of stabilized polymer crystal fragments occurred. The concentration of the crystal fragments varies in the Ts1–Ts2 range and it increases as Ts decreases reaching the saturation for Ts = Ts2.
- Final crystallization (Tc2): in this last step, a second crystallization is achieved by cooling the sample by 10 °C/min to 65 °C. At this point, the crystallization peak will be located at Tc2 (with Tc2 ≥ Tc1). This Tc increment is correlated to an increment of the nucleation site concentration induced by the self-nucleation process. Consequently, the PLA sample (not self-nucleated) crystallizes at the lowest temperature, Tc1, whereas the best self-nucleated samples crystallizes at the highest temperature, Tc2,max.
2.4. Mechanical Characterization
2.5. Heat Defection Temperature (HDT) Measurements
3. Results and Discussion
3.1. Identification of the Best Nucleating Agent Content
3.2. Injection Molded Specimen Results
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okada, M. Chemical syntheses of biodegradable polymers. Prog. Polym. Sci. 2002, 27, 87–133. [Google Scholar] [CrossRef]
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic acid (PLA) synthesis and modifications: A review. Front. Chem. China 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Pietrosanto, A.; Scarfato, P.; Di Maio, L.; Nobile, M.R.; Incarnato, L. Evaluation of the Suitability of Poly(Lactide)/Poly(Butylene-Adipate-co-Terephthalate) Blown Films fro Chilled and Frozen Food Packaging Applications. Polymers 2020, 12, 804. [Google Scholar] [CrossRef] [Green Version]
- Narancic, T.; Cerrone, F.; Beagan, N. Recent Advances in Bioplastics: Application and Biodegradation. Polymers 2020, 12, 920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusu, D.; Boyer, S.; Lacrampe, M.F.; Krawczak, P. Bioplastics for automotive applications. In Handbook of Bioplastics and Biocomposites Engineering Applications; John Wiley and Sons: Hoboken, NJ, USA, 2011; Volume 81, p. 397. [Google Scholar]
- Coltelli, M.-B.; Gigante, V.; Cinelli, P.; Vannozzi, A.; Aliotta, L.; Lazzeri, A. Biobased and biodegradable rigid and flexible polymeric packaging. In An Introduction to the Circular Economy; Nova Science Publisher, Inc.: Hauppauge, NY, USA, 2021; pp. 365–390. ISBN 978-1-53619-233-9. [Google Scholar]
- Ray, S.S.; Bousmina, M. Biodegradable polymers and their layered silicate nanocomposites: In greening the 21st century materials world. Prog. Mater. Sci. 2005, 50, 962–1079. [Google Scholar]
- Gruber, P.R.; Drumright, R.E.; Henton, D.E. Polylactic acid technology. Adv. Mater 2000, 12, 1841–1846. [Google Scholar]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, V.; Zhang, K.; Misra, M.; Mohanty, A.K. Overcoming the fundamental challenges in improving the impact strength and crystallinity of PLA biocomposites: Influence of nucleating agent and mold temperature. ACS Appl. Mater. Interfaces 2015, 7, 11203–11214. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Effect of nucleation and plasticization on the crystallization of poly(lactic acid). Polymer 2007, 48, 6855–6866. [Google Scholar] [CrossRef] [Green Version]
- Aliotta, L.; Gazzano, M.; Lazzeri, A.; Righetti, M.C. Constrained Amorphous Interphase in Poly (L -lactic acid): Estimation of the Tensile Elastic Modulus. ACS Omega 2020, 5, 20890–20902. [Google Scholar] [CrossRef] [PubMed]
- Cocca, M.; Di Lorenzo, M.L.; Malinconico, M.; Frezza, V. Influence of crystal polymorphism on mechanical and barrier properties of poly(l-lactic acid). Eur. Polym. J. 2011, 47, 1073–1080. [Google Scholar] [CrossRef]
- Aliotta, L.; Cinelli, P.; Coltelli, M.B.; Righetti, M.C.; Gazzano, M.; Lazzeri, A. Effect of nucleating agents on crystallinity and properties of poly (lactic acid) (PLA). Eur. Polym. J. 2017, 93, 822–832. [Google Scholar] [CrossRef]
- Pan, P.; Inoue, Y. Polymorphism and isomorphism in biodegradable polyesters. Prog. Polym. Sci. 2009, 34, 605–640. [Google Scholar] [CrossRef]
- Kawai, T.; Rahman, N.; Matsuba, G.; Nishida, K.; Kanaya, T.; Nakano, M.; Okamoto, H.; Kawada, J.; Usuki, A.; Honma, N.; et al. Crystallization and melting behavior of poly (L-lactic acid). Macromolecules 2007, 40, 9463–9469. [Google Scholar] [CrossRef]
- Androsch, R.; Schick, C.; Lorenzo, M.L. Di Melting of Conformationally Disordered Crystals (α′-Phase) of Poly(l-lactic acid). Macromol. Chem. Phys. 2014, 215, 1134–1139. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Disorder-to-order phase transition and multiple melting behavior of poly(L-lactide) investigated by simultaneous measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Pan, P.; Zhu, B.; Kai, W.; Dong, T.; Inoue, Y. Effect of crystallization temperature on crystal modifications and crystallization kinetics of poly (L-lactide). J. Appl. Polym. Sci. 2008, 107, 54–62. [Google Scholar] [CrossRef]
- Navrátilová, N.; Náplava, A. Study of Biodegradable Plastics Produced By Injection Molding. Mater. Sci. Technol. 2011, 11, 48–53. [Google Scholar]
- Dang, X.P. General frameworks for optimization of plastic injection molding process parameters. Simul. Model. Pract. Theory 2014, 41, 15–27. [Google Scholar] [CrossRef]
- Suryanegara, L.; Okumura, H.; Nakagaito, A.N.; Yano, H. The synergetic effect of phenylphosphonic acid zinc and microfibrillated cellulose on the injection molding cycle time of PLA composites. Cellulose 2011, 18, 689–698. [Google Scholar] [CrossRef]
- Kfoury, G.; Raquez, J.-M.; Hassouna, F.; Odent, J.; Toniazzo, V.; Ruch, D.; Dubois, P. Recent advances in high performance poly (lactide): From “green” plasticization to super-tough materials via (reactive) compounding. Front. Chem. 2013, 1, 32. [Google Scholar] [CrossRef] [Green Version]
- Mathew, A.P.; Oksman, K.; Sain, M. The effect of morphology and chemical characteristics of cellulose reinforcements on the crystallinity of polylactic acid. J. Appl. Polym. Sci. 2006, 101, 300–310. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J. Research progress in toughening modification of poly(lactic acid). J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1051–1083. [Google Scholar] [CrossRef]
- Ljungberg, N.; Wesslen, B. The effects of plasticizers on the dynamic mechanical and thermal properties of poly (lactic acid). J. Appl. Polym. Sci. 2002, 86, 1227–1234. [Google Scholar] [CrossRef]
- Harris, A.M.; Lee, E.C. Improving mechanical performance of injection molded PLA by controlling crystallinity. J. Appl. Polym. Sci. 2008, 107, 2246–2255. [Google Scholar] [CrossRef]
- Kawamoto, N.; Sakai, A.; Horikoshi, T.; Urushihara, T.; Tobita, E. Nucleating agent for poly (l-lactic acid)—An optimization of chemical structure of hydrazide compound for advanced nucleation ability. J. Appl. Polym. Sci. 2007, 103, 198–203. [Google Scholar] [CrossRef]
- Tsuji, H.; Takai, H.; Saha, S.K. Isothermal and non-isothermal crystallization behavior of poly (L-lactic acid): Effects of stereocomplex as nucleating agent. Polymer 2006, 47, 3826–3837. [Google Scholar] [CrossRef]
- Nam, J.Y.; Okamoto, M.; Okamoto, H.; Nakano, M.; Usuki, A.; Matsuda, M. Morphology and crystallization kinetics in a mixture of low-molecular weight aliphatic amide and polylactide. Polymer 2006, 47, 1340–1347. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Feng, Y.; Ma, P.; Xu, P.; Wang, R.; Dong, W.; Chen, M.; Joziasse, C. The crystallization behavior of poly(lactic acid) with different types of nucleating agents. Int. J. Biol. Macromol. 2018, 106, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Aliotta, L.; Gigante, V.; Coltelli, M.; Cinelli, P.; Lazzeri, A.; Seggiani, M. Thermo-Mechanical Properties of PLA/Short Flax Fiber Biocomposites. Appl. Sci. 2019, 9, 3797. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tong, B.; Hou, S.; Li, M.; Shen, C. Transcrystallization behavior at the poly (lactic acid)/sisal fiber biocomposite interface. Compos. Part A Appl. Sci. Manuf. 2011, 42, 66–74. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Huang, Z.; Weng, Y. Heat resistance, crystallization behavior, and mechanical properties of polylactide/nucleating agent composites. Mater. Des. 2015, 66, 7–15. [Google Scholar] [CrossRef]
- Schäfer, H.; Pretschuh, C.; Brüggemann, O. Reduction of cycle times in injection molding of PLA through bio-based nucleating agents. Eur. Polym. J. 2019, 115, 6–11. [Google Scholar] [CrossRef]
- Ageyeva, T.; Kovács, J.G.; Tábi, T. Comparison of the efficiency of the most effective heterogeneous nucleating agents for Poly(lactic acid). J. Therm. Anal. Calorim. 2021. [Google Scholar] [CrossRef]
- Petchwattana, N.; Narupai, B. Synergistic Effect of Talc and Titanium Dioxide on Poly(lactic acid) Crystallization: An Investigation on the Injection Molding Cycle Time Reduction. J. Polym. Environ. 2019, 27, 837–846. [Google Scholar] [CrossRef]
- Wei, Z.; Shao, S.; Sui, M.; Song, P.; He, M.; Xu, Q.; Leng, X.; Wang, Y.; Li, Y. Development of zinc salts of amino acids as a new class of biocompatible nucleating agents for poly(L-lactide). Eur. Polym. J. 2019, 118, 337–346. [Google Scholar] [CrossRef]
- Gong, X.; Pan, L.; Tang, C.Y.; Chen, L.; Li, C.; Wu, C.; Law, W.C.; Wang, X.; Tsui, C.P.; Xie, X. Investigating the crystallization behavior of poly(lactic acid) using CdSe/ZnS quantum dots as heterogeneous nucleating agents. Compos. Part B Eng. 2016, 91, 103–110. [Google Scholar] [CrossRef]
- Tang, H.; Chen, J.-B.; Wang, Y.; Xu, J.Z.; Hsiao, B.S.; Zhong, G.J.; Li, Z.M. Shear flow and carbon nanotubes synergistically induced nonisothermal crystallization of poly(lactic acid) and its application in injection molding. Biomacromolecules 2012, 13, 3858–3867. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. II transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Wang, Y.; He, D.; Wang, X.; Cao, W.; Li, Q.; Shen, C. Crystallization of poly(lactic acid) enhanced by phthalhydrazide as nucleating agent. Polym. Bull. 2013, 70, 2911–2922. [Google Scholar] [CrossRef]
- He, D.; Wang, Y.; Shao, C.; Zheng, G.; Li, Q.; Shen, C. Effect of phthalimide as an efficient nucleating agent on the crystallization kinetics of poly(lactic acid). Polym. Test. 2013, 32, 1088–1093. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid-Z. Z. Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- Fillon, B.; Thierry, A.; Lotz, B.; Wittmann, J.C. Efficiency scale for polymer nucleating agents. J. Therm. Anal. 1994, 42, 721–731. [Google Scholar] [CrossRef]
- Fillon, B.; Wittmann, J.C.; Lotz, B.; Thierry, A. Self-nucleation and recrystallization of isotactic polypropylene (α phase) investigated by differential scanning calorimetry. J. Polym. Sci. Part B Polym. Phys. 1993, 31, 1383–1393. [Google Scholar] [CrossRef]
- Schmidt, S.C.; Hillmyer, M.A. Polylactide Stereocomplex Crystallites as Nucleating Agents for Isotactic Polylactide. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 300–313. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, C.; Liu, X.; Zhu, J. The crystallization behavior and mechanical properties of polylactic acid in the presence of a crystal nucleating agent. J. Appl. Polym. Sci. 2012, 125, 1108–1115. [Google Scholar] [CrossRef]
- Aliotta, L.; Vannozzi, A.; Canesi, I.; Cinelli, P.; Coltelli, M.; Lazzeri, A. Poly(lactic acid) (PLA)/Poly(butylene succinate-co-adipate) (PBSA) Compatibilized Binary Biobased Blends: Melt Fluidity, Morphological, Thermo-Mechanical and Micromechanical Analysis. Polymers 2021, 13, 218. [Google Scholar] [CrossRef]
- Jalali, A.; Huneault, M.A.; Elkoun, S. Effect of molecular weight on the nucleation efficiency of poly(lactic acid) crystalline phases. J. Polym. Res. 2017, 24, 182. [Google Scholar] [CrossRef]
- Kovalcik, A.; Pérez-Camargo, R.A.; Fürst, C.; Kucharczyk, P.; Müller, A.J. Nucleating efficiency and thermal stability of industrial non-purified lignins and ultrafine talc in poly(lactic acid) (PLA). Polym. Degrad. Stab. 2017, 142, 244–254. [Google Scholar] [CrossRef]
- Pyda, M.; Bopp, R.C.; Wunderlich, B. Heat capacity of poly (lactic acid). J. Chem. Thermodyn. 2004, 36, 731–742. [Google Scholar] [CrossRef]
- Aliotta, L.; Cinelli, P.; Beatrice Coltelli, M.; Lazzeri, A. Rigid filler toughening in PLA-Calcium Carbonate composites: Effect of particle surface treatment and matrix plasticization. Eur. Polym. J. 2018, 113, 78–88. [Google Scholar] [CrossRef]









| Name | PLA (wt %) | LAK (wt %) | Talc (wt %) | CaCO3 (wt %) |
|---|---|---|---|---|
| PLA | 100 | - | - | - |
| PLA_L_1 | 99 | 1 | - | - |
| PLA_L_3 | 97 | 3 | - | - |
| PLA_L_5 | 95 | 5 | - | - |
| PLA_T_1 | 99 | - | 1 | - |
| PLA_T_3 | 97 | - | 3 | - |
| PLA_T_5 | 95 | - | 5 | - |
| PLA_C_1 | 99 | - | - | 1 |
| PLA_C_3 | 97 | - | - | 3 |
| PLA_C_5 | 95 | - | - | 5 |
| Main Injection Molding Parameters | |
|---|---|
| Temperature profile (°C) | 180/185/190/190 |
| Mold temperature (°C) | 110 |
| Injection and holding time (s) | 5 |
| Injection pressure (bar) | 70 |
| Cooling time (s) | 25/55 |
| Blend Name | Cycle time (s) | Tg (°C) | Tcc (°C) | Tm (°C) | ΔHcc (J/g) | ΔHm (J/g) | Xcc (%) |
|---|---|---|---|---|---|---|---|
| PLA | 30 | 58.6 | 98.6 | 176.4 | 29.6 | 47.5 | 19.2 |
| PLA | 60 | 58.5 | 97.9 | 176.9 | 23.9 | 44 | 21.5 |
| PLA_L_3 | 30 | 58.7 | - | 177.7 | - | 47.1 | 52.2 |
| PLA_L_3 | 60 | 58.8 | - | 177.7 | - | 50.4 | 55.9 |
| PLA_T_3 | 30 | 58.9 | 90 | 177.4 | 13.3 | 50.3 | 41 |
| PLA_T_3 | 60 | 58.5 | - | 177.7 | - | 48.1 | 53.3 |
| PLA_C_1 | 30 | 58.8 | 93.8 | 176.8 | 28.6 | 49 | 22.2 |
| PLA_C_1 | 60 | 58.6 | 91.4 | 177 | 14.7 | 48.4 | 36.6 |
| Blend Name | Cycle Time (s) | Young Modulus (GPa) | Tensile Stress (MPa) | Elongation at Break (%) | Flexural Modulus (GPa) | Charpy Impact Strength C.I.S. (kJ/m2) | HDT (°C) |
|---|---|---|---|---|---|---|---|
| PLA | 30 | 3.45 ± 0.09 | 59.20 ± 0.72 | 3.22 ± 0.44 | 4.17 ± 0.12 | 2.81 ± 0.28 | 55.4 ± 0.5 |
| PLA | 60 | 3.53 ± 0.03 | 60.30 ± 0.43 | 2.65 ± 0.10 | 4.40 ± 0.07 | 2.66 ± 0.13 | 55.5 ± 0.6 |
| PLA_L_3 | 30 | 4.38 ± 0.01 | 53.07 ± 0.40 | 1.88 ± 0.07 | 5.24 ± 0.08 | 2.76 ± 0.25 | 129.7 ± 1.3 |
| PLA_L_3 | 60 | 4.35 ± 0.03 | 54.32 ± 0.46 | 1.89 ± 0.05 | 5.43 ±0.12 | 2.56 ± 0.36 | 142.0 ± 1.1 |
| PLA_T_3 | 30 | 4.23 ± 0.09 | 58.32 ± 0.94 | 2.33 ± 0.31 | 4.73 ± 0.11 | 3.92 ± 0.65 | 65.7 ± 0.9 |
| PLA_T_3 | 60 | 4.52 ± 0.05 | 58.6 ± 0.41 | 1.85 ± 0.11 | 5.3 ± 0.06 | 3.78 ± 0.19 | 137.6 ± 1.1 |
| PLA_C_1 | 30 | 3.51 ± 0.09 | 58.92 ± 0.53 | 2.72 ± 0.18 | 4.16 ± 0.13 | 3.10 ± 0.62 | 55.7 ± 0.3 |
| PLA_C_1 | 60 | 3.62 ± 0.07 | 59.96 ± 0.65 | 2.46 ± 0.12 | 4.53 ± 0.03 | 3.00 ± 0.65 | 61.4 ± 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliotta, L.; Sciara, L.M.; Cinelli, P.; Canesi, I.; Lazzeri, A. Improvement of the PLA Crystallinity and Heat Distortion Temperature Optimizing the Content of Nucleating Agents and the Injection Molding Cycle Time. Polymers 2022, 14, 977. https://doi.org/10.3390/polym14050977
Aliotta L, Sciara LM, Cinelli P, Canesi I, Lazzeri A. Improvement of the PLA Crystallinity and Heat Distortion Temperature Optimizing the Content of Nucleating Agents and the Injection Molding Cycle Time. Polymers. 2022; 14(5):977. https://doi.org/10.3390/polym14050977
Chicago/Turabian StyleAliotta, Laura, Letizia Maria Sciara, Patrizia Cinelli, Ilaria Canesi, and Andrea Lazzeri. 2022. "Improvement of the PLA Crystallinity and Heat Distortion Temperature Optimizing the Content of Nucleating Agents and the Injection Molding Cycle Time" Polymers 14, no. 5: 977. https://doi.org/10.3390/polym14050977
APA StyleAliotta, L., Sciara, L. M., Cinelli, P., Canesi, I., & Lazzeri, A. (2022). Improvement of the PLA Crystallinity and Heat Distortion Temperature Optimizing the Content of Nucleating Agents and the Injection Molding Cycle Time. Polymers, 14(5), 977. https://doi.org/10.3390/polym14050977