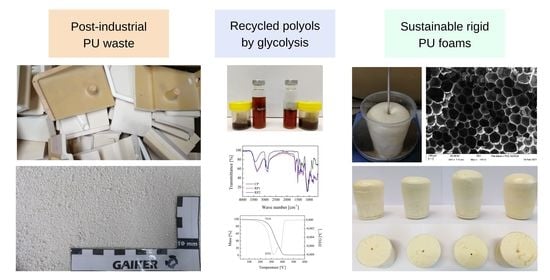
Synthesis of Rigid Polyurethane Foams Incorporating Polyols from Chemical Recycling of Post-Industrial Waste Polyurethane Foams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Glycolysis of Waste RPUFs and Purification
2.3. Synthesis of New RPUFs
2.4. Characterization of Polyols
2.5. Characterization of RPUFs
3. Results and Discussion
3.1. Analysis and Characteristics of the Polyols
3.2. Synthesis of PU Foams Based on Recycled Polyols
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gama, N.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randall, D.; Lee, S. Introduction to Polyurethanes. In The Polyurethanes Book; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Szycher, M. Basic Concepts in Polyurethane Chemistry and Technology. In Szycher’s Handbook of Polyurethanes; CRC Press: New York, NY, USA, 2012. [Google Scholar]
- Palm, E.; Svensson Myrin, E. Mapping the Plastics System and Its Sustainability Challenges; Lund University: Lund, Sweden, 2018. [Google Scholar]
- Yang, W.; Dong, Q.; Liu, S.; Xie, H.; Liu, L.; Li, J. Recycling and Disposal Methods for Polyurethane Foam Wastes. Procedia Environ. Sci. 2012, 16, 167–175. [Google Scholar] [CrossRef]
- Zia, K.M.; Bhatti, H.N.; Ahmad Bhatti, I. Methods for polyurethane and polyurethane composites, recycling and recovery: A review. React. Funct. Polym. 2007, 67, 675–692. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.; de Lucas, A.; Rodríguez, J. Recycling of polyurethanes from laboratory to industry, a journey towards the sustainability. Waste Manag. 2018, 76, 147–171. [Google Scholar] [CrossRef]
- Wu, C.H.; Chang, C.Y.; Cheng, C.M.; Huang, H.C. Glycolysis of waste flexible polyurethane foam. Polym. Degrad. Stab. 2003, 80, 103–111. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Czupryński, B.; Liszkowska, J. Glycolysis of rigid polyurethane–polyisocyanurate foams with reduced flammability. J. Elastomers Plast. 2016, 48, 340–353. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.; de Lucas, A.; Rodríguez, J. Glycolysis of viscoelastic flexible polyurethane foam wastes. Polym. Degrad. Stab. 2015, 116, 23–35. [Google Scholar] [CrossRef]
- Watando, H.; Saya, S.; Fukaya, T.; Fujieda, S.; Yamamoto, M. Improving chemical recycling rate by reclaiming polyurethane elastomer from polyurethane foam. Polym. Degrad. Stab. 2006, 91, 3354–3359. [Google Scholar] [CrossRef]
- Kanaya, K.; Takahashi, S. Decomposition of polyurethane foams by alkanolamines. J. Appl. Polym. Sci. 1994, 51, 675–682. [Google Scholar] [CrossRef]
- Chuayjuljit, S.; Norakankorn, C.; Pimpan, V. Chemical recycling of rigid polyurethane foam scrap via base catalyzed aminolysis. J. Met. Mater. Miner. 2002, 12, 19–22. [Google Scholar]
- Behrendt, G.; Naber, B.W. The chemical recycling of polyurethanes. J. Univ. Chem. Technol. Metall. 2009, 44, 3–23. [Google Scholar]
- Li, C.; Liu, L.; Zhu, C. Characterization of Renewable PUF and Preparation of Polyurethane Foam Composites with Alkali Lignin/Renewable PUF. Open Mater. Sci. J. 2011, 5, 130–133. [Google Scholar] [CrossRef] [Green Version]
- Motokucho, S.; Nakayama, Y.; Morikawa, H.; Nakatani, H. Environment-friendly chemical recycling of aliphatic polyurethanes by hydrolysis in a CO2-water system. J. Appl. Polym. Sci. 2018, 135, 45897. [Google Scholar] [CrossRef]
- Yamamoto, N.; Nakayama, A.; Oshima, M.; Kawasaki, N.; Aiba, S. Enzymatic hydrolysis of lysine diisocyanate based polyurethanes and segmented polyurethane ureas by various proteases. React. Funct. Polym. 2007, 67, 1338–1345. [Google Scholar] [CrossRef]
- Morooka, H.; Nakakawaji, T.; Okamoto, S.; Araki, K.; Yamada, E. Chemical recycling of rigid polyurethane foam for refrigerators. Polym. Prepr. 2005, 54, 1951. [Google Scholar]
- Nikje, M.; Nikrah, M. Chemical Recycling and Liquefaction of Rigid Polyurethane Foam Wastes through Microwave Assisted Glycolysis Process. J. Macromol. Sci. A 2007, 44, 613–617. [Google Scholar] [CrossRef]
- Zhu, P.; Cao, Z.; Chen, Y.; Zhang, X.; Qian, G.; Chu, Y.; Zhou, M. Glycolysis recycling of rigid waste polyurethane foam from refrigerators. Environ. Technol. 2014, 35, 2676–2684. [Google Scholar] [CrossRef]
- Li, Q.; Xu, W.; Xi, E.; Pang, Y.; Liu, X.; Mao, A. Recovery of Polyols from Polyurethane Foam Wastes by Solvent Decomposition. IOP Conf. Ser. Earth Environ. Sci. 2019, 310, 042014. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Chang, C.; Li, J. Glycolysis of rigid polyurethane from waste refrigerators. Polym. Degrad. Stab. 2002, 75, 413–421. [Google Scholar] [CrossRef]
- Shin, S.; Kim, H.; Liang, J.; Lee, S.; Lee, D. Sustainable rigid polyurethane foams based on recycled polyols from chemical recycling of waste polyurethane foams. J. Appl. Polym. Sci. 2019, 136, 47916. [Google Scholar] [CrossRef]
- Murai, M.; Sanou, M.; Fujimoto, T.; Baba, F. Glycolysis of Rigid Polyurethane Foam under Various Reaction Conditions. J. Cell. Plast. 2003, 39, 15–27. [Google Scholar] [CrossRef]
- Thomas, S.; Rane, A.; Kanny, K.; Abitha, V.K.; Thomas, M. Polyurethane Foam Chemistry. In Recycling of Polyurethane Foams; William Andrew: Oxford, UK, 2018. [Google Scholar]
- Zhang, L.; Zhang, M.; Hu, L.; Zhou, Y. Synthesis of Rigid Polyurethane Foams with Castor Oil-Based Flame Retardant Polyols. Ind. Crops Prod. 2014, 52, 380–388. [Google Scholar] [CrossRef]
- Simón, D.; de Lucas, A.; Rodríguez, J.; Borreguero, A. Flexible polyurethane foams synthesized employing recovered polyols from glycolysis: Physical and structural properties. J. Appl. Polym. Sci. 2017, 134, 45087. [Google Scholar] [CrossRef]
- Ionescu, M. Polyols from Renewable Resources. In Chemistry and Technology of Polyols for Polyurethanes; Rapra Technology: Shropshire, UK, 2016. [Google Scholar]
- Abril-Milán, D.; Valdés, O.; Mirabal-Gallardo, Y.; de la Torre, A.F.; Bustamante, C.; Contreras, J. Preparation of Renewable Bio-Polyols from Two Species of Colliguaja for Rigid Polyurethane Foams. Materials 2018, 11, 2244. [Google Scholar] [CrossRef] [Green Version]
- Kraitape, N.; Thongpin, C. Influence of Recycled Polyurethane Polyol on the Properties of Flexible Polyurethane Foams. Energy Procedia 2016, 89, 186–197. [Google Scholar] [CrossRef] [Green Version]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef] [Green Version]
- Kurańska, M.; Pinto, J.; Salach, K.; Barreiro, M.; Prociak, A. Synthesis of thermal insulating polyurethane foams from lignin and rapeseed based polyols: A comparative study. Ind. Crops Prod. 2020, 143, 111882. [Google Scholar] [CrossRef]
- Dhaliwal, G.S.; Anandan, S.; Chandrashekhara, K.; Dudenhoeffer, N.; Nam, P. Fabrication and testing of soy-based polyurethane foam for insulation and structural applications. J. Polym. Environ. 2019, 27, 1897–1907. [Google Scholar] [CrossRef]
- Dhaliwal, G.S.; Anandan, S.; Bose, M.; Chandrashekhara, K.; Nam, P. Effects of surfactants on mechanical and thermal properties of soy-based polyurethane foams. J. Cell. Plast. 2020, 56, 611–629. [Google Scholar] [CrossRef]









| Recycled Polyol Code | Solvent | Catalyst | Solvent:PU [g:g] | Catalyst:PU [mol:g] | Temperature [°C] | Time [h] |
|---|---|---|---|---|---|---|
| RP1 | EG | NaOH | 4:1 | 0.002:1 | 198 | 2 |
| RP2 | EG | NaOH | 1.5:1 | 0.002:1 | 180 | 2 |
| Sample | COM | RP1-5 | RP1-10 | RP1-15 | RP2-5 | RP2-10 | RP2-15 |
|---|---|---|---|---|---|---|---|
| Component A [php] 1 | |||||||
| CP | 100 | 95 | 90 | 85 | 95 | 90 | 85 |
| RP1 | - | 5 | 10 | 15 | - | - | - |
| RP2 | - | - | - | - | 5 | 10 | 15 |
| Water | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| DMAE | 1 | - | - | - | - | - | - |
| Component B | |||||||
| NCO index | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 |
| Polyol Code | Hydroxyl Value [mg KOH g−1] | Acid Value [mg KOH g−1] | Mn (g/mol) | Mw/Mn | Viscosity @25 °C [Pa·s] |
|---|---|---|---|---|---|
| RP1 | 940 ± 85 | 8.7 ± 0.09 | 873.8 ± 32 | 1.031 ± 0.04 | 17,820 ± 970 |
| RP2 | 1130 ± 95 | 4.48 ± 0.42 | 885.4 ± 35 | 1.037 ± 0.04 | 10,720 ± 610 |
| CP | 395 ± 65 | 0.09 ± 0.02 | 535.5 ± 21 | 1.014 ± 0.04 | 1690 ± 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amundarain, I.; Miguel-Fernández, R.; Asueta, A.; García-Fernández, S.; Arnaiz, S. Synthesis of Rigid Polyurethane Foams Incorporating Polyols from Chemical Recycling of Post-Industrial Waste Polyurethane Foams. Polymers 2022, 14, 1157. https://doi.org/10.3390/polym14061157
Amundarain I, Miguel-Fernández R, Asueta A, García-Fernández S, Arnaiz S. Synthesis of Rigid Polyurethane Foams Incorporating Polyols from Chemical Recycling of Post-Industrial Waste Polyurethane Foams. Polymers. 2022; 14(6):1157. https://doi.org/10.3390/polym14061157
Chicago/Turabian StyleAmundarain, Izotz, Rafael Miguel-Fernández, Asier Asueta, Sara García-Fernández, and Sixto Arnaiz. 2022. "Synthesis of Rigid Polyurethane Foams Incorporating Polyols from Chemical Recycling of Post-Industrial Waste Polyurethane Foams" Polymers 14, no. 6: 1157. https://doi.org/10.3390/polym14061157
APA StyleAmundarain, I., Miguel-Fernández, R., Asueta, A., García-Fernández, S., & Arnaiz, S. (2022). Synthesis of Rigid Polyurethane Foams Incorporating Polyols from Chemical Recycling of Post-Industrial Waste Polyurethane Foams. Polymers, 14(6), 1157. https://doi.org/10.3390/polym14061157