Continuous Nanoprecipitation of Polycaprolactone in Additively Manufactured Micromixers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Construction and Printing of Micromixers
2.3. Discontinuous Nanoparticle Formulation
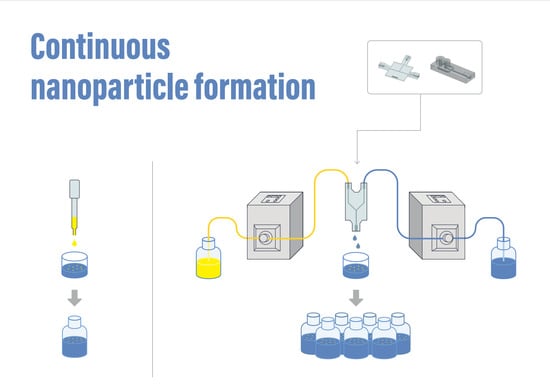
2.4. Continuous Nanoparticle Formulation
2.5. Particles Size Analysis
2.6. Zeta Potential
2.7. Atomic Force Microscopy
3. Results and Discussion
3.1. Discontinuous Process for the Formulation of PCL Nanoparticles
3.2. Continuous Process for the Formulation of PCL Nanoparticles
3.3. Effect of Solvent Evaporation
3.4. Effect of Surfactant
3.5. Encapsulation of Fluorescence Dye Nile Red
3.6. Role of the Micromixer
3.7. Atomic Force Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aschenbrenner, E.; Bley, K.; Koynov, K.; Makowski, M.; Kappl, M.; Landfester, K.; Weiss, C.K. Using the polymeric ouzo effect for the preparation of polysaccharide-based nanoparticles. Langmuir 2013, 29, 8845–8855. [Google Scholar] [CrossRef] [PubMed]
- Barthel, A.K.; Dass, M.; Droge, M.; Cramer, J.M.; Baumann, D.; Urban, M.; Landfester, K.; Mailander, V.; Lieberwirth, I. Imaging the intracellular degradation of biodegradable polymer nanoparticles. Beilstein J. Nanotechnol. 2014, 5, 1905–1917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bovone, G.; Steiner, F.; Guzzi, E.A.; Tibbitt, M.W. Automated and continuous production of polymeric nanoparticles. Front. Bioeng. Biotechnol. 2019, 7, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, X.; Tang, H.; Mao, H.Q. Effective encapsulation of curcumin in nanoparticles enabled by hydrogen bonding using flash nanocomplexation. Int. J. Pharm. 2019, 564, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Greatti, V.R.; Oda, F.; Sorrechia, R.; Kapp, B.R.; Seraphim, C.M.; Weckwerth, A.; Chorilli, M.; Silva, P.B.D.; Eloy, J.O.; Kogan, M.J.; et al. Poly-ε-caprolactone nanoparticles loaded with 4-nerolidylcatechol (4-NC) for growth inhibition of microsporum canis. Antibiotics 2020, 9, 894. [Google Scholar] [CrossRef]
- Loos, C.; Syrovets, T.; Musyanovych, A.; Mailander, V.; Landfester, K.; Nienhaus, G.U.; Simmet, T. Functionalized polystyrene nanoparticles as a platform for studying bio-nano interactions. Beilstein J. Nanotechnol. 2014, 5, 2403–2412. [Google Scholar] [CrossRef] [Green Version]
- Martinez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodriguez, S.A.; Roman, R.A.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef]
- Dausend, J.; Musyanovych, A.; Dass, M.; Walther, P.; Schrezenmeier, H.; Landfester, K.; Mailander, V. Uptake mechanism of oppositely charged fluorescent nanoparticles in HeLa cells. Macromol. Biosci. 2008, 8, 1135–1143. [Google Scholar] [CrossRef]
- Lepeltier, E.; Bourgaux, C.; Couvreur, P. Nanoprecipitation and the “ouzo effect”: Application to drug delivery devices. Adv. Drug Deliv. Rev. 2014, 71, 86–97. [Google Scholar] [CrossRef]
- Yan, X.; Delgado, M.; Fu, A.; Alcouffe, P.; Gouin, S.G.; Fleury, E.; Katz, J.L.; Ganachaud, F.; Bernard, J. Simple but precise engineering of functional nanocapsules through nanoprecipitation. Angew. Chem. Int. Ed. Engl. 2014, 53, 6910–6913. [Google Scholar] [CrossRef]
- Vitale, S.A.; Katz, J.L. Liquid droplet dispersions formed by homogeneous liquid−liquid nucleation: “The ouzo effect”. Langmuir 2003, 19, 4105–4110. [Google Scholar] [CrossRef]
- Weiss, C.K.; Lorenz, M.R.; Landfester, K.; Mailander, V. Cellular uptake behavior of unfunctionalized and functionalized PBCA particles prepared in a miniemulsion. Macromol. Biosci. 2007, 7, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Badri, W.; Miladi, K.; Nazari, Q.A.; Fessi, H.; Elaissari, A. Effect of process and formulation parameters on polycaprolactone nanoparticles prepared by solvent displacement. Colloids Surf. A Physicochem. 2017, 516, 238–244. [Google Scholar] [CrossRef]
- Botet, R. The “ouzo effect”, recent developments and application to therapeutic drug carrying. J. Phys. Conf. Ser. 2012, 352. [Google Scholar] [CrossRef]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Influence of process and formulation parameters on the formation of submicron particles by solvent displacement and emulsification-diffusion methods critical comparison. Adv. Colloid Interface Sci. 2011, 163, 90–122. [Google Scholar] [CrossRef]
- Pucci, C.; Cousin, F.; Dole, F.; Chapel, J.-P.; Schatz, C. Impact of the formulation pathway on the colloidal state and crystallinity of poly-ε-caprolactone particles prepared by solvent displacement. Langmuir 2018, 34, 2531–2542. [Google Scholar] [CrossRef]
- Quintanar-Guerrero, D.; Fessi, H.; Allémann, E.; Doelker, E. Influence of stabilizing agents and preparative variables on the formation of poly(d,l-lactic acid) nanoparticles by an emulsification-diffusion technique. Int. J. Pharm. 1996, 143, 133–141. [Google Scholar] [CrossRef]
- Lammari, N.; Louaer, O.; Meniai, A.H.; Elaissari, A. Encapsulation of essential oils via nanoprecipitation process: Overview, progress, challenges and prospects. Pharmaceutics 2020, 12, 431. [Google Scholar] [CrossRef]
- Bhargava, S.; Chu, J.J.H.; Valiyaveettil, S. Controlled dye aggregation in sodium dodecylsulfate-stabilized poly(methylmethacrylate) nanoparticles as fluorescent imaging probes. ACS Omega 2018, 3, 7663–7672. [Google Scholar] [CrossRef] [Green Version]
- Verkhovskii, R.; Kozlova, A.; Atkin, V.; Kamyshinsky, R.; Shulgina, T.; Nechaeva, O. Physical properties and cytotoxicity of silver nanoparticles under different polymeric stabilizers. Heliyon 2019, 5, e01305. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Shah, K.W.; Xu, J. Synthesis, morphologies and building applications of nanostructured polymers. Polymers 2017, 9, 506. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, A.L.; Trivedi, V.; Mitchell, J.C. Preparation of polycaprolactone nanoparticles via supercritical carbon dioxide extraction of emulsions. Drug Deliv. Transl. Res. 2018, 8, 1790–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Markwalter, C.E.; Tian, C.; Armstrong, M.; Prud’homme, R.K. Translational formulation of nanoparticle therapeutics from laboratory discovery to clinical scale. J. Transl. Med. 2019, 17, 200. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.F.; Wan, K.Y.; Cheng, K.K.; Wong, K.W.; Sun, C.C.; Baum, L.; Chow, A.H.L. Development of highly stabilized curcumin nanoparticles by flash nanoprecipitation and lyophilization. Eur. J. Pharm. Biopharm. 2015, 94, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Pansare, V.J.; Prud’homme, R.K.; Priestley, R.D. Flash nanoprecipitation of polystyrene nanoparticles. Soft Matter 2012, 8, 86–93. [Google Scholar] [CrossRef]
- Ferri, A.; Kumari, N.; Peila, R.; Barresi, A.A. Production of menthol-loaded nanoparticles by solvent displacement. Can. J. Chem. Eng. 2017, 95, 1690–1706. [Google Scholar] [CrossRef]
- Valente, I.; Celasco, E.; Marchisio, D.L.; Barresi, A.A. Nanoprecipitation in confined impinging jets mixers: Production, characterization and scale-up of pegylated nanospheres and nanocapsules for pharmaceutical use. Chem. Eng. Sci. 2012, 77, 217–227. [Google Scholar] [CrossRef]
- Zelenkova, T.; Fissore, D.; Marchisio, D.L.; Barresi, A.A. Size control in production and freeze-drying of poly-epsilon-caprolactone nanoparticles. J. Pharm. Sci. 2014, 103, 1839–1850. [Google Scholar] [CrossRef]
- Campana, A.L.; Sotelo, D.C.; Oliva, H.A.; Aranguren, A.; Ornelas-Soto, N.; Cruz, J.C.; Osma, J.F. Fabrication and characterization of a low-cost microfluidic system for the manufacture of alginate-lacasse microcapsules. Polymers 2020, 12, 1158. [Google Scholar] [CrossRef]
- Aranguren, A.; Torres, C.E.; Munoz-Camargo, C.; Osma, J.F.; Cruz, J.C. Synthesis of nanoscale liposomes via low-cost microfluidic systems. Micromachines 2020, 11, 50. [Google Scholar] [CrossRef]
- Lince, F.; Marchisio, D.L.; Barresi, A.A. Strategies to control the particle size distribution of poly-epsilon-caprolactone nanoparticles for pharmaceutical applications. J. Colloid Interface Sci. 2008, 322, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Lince, F.; Marchisio, D.L.; Barresi, A.A. Smart mixers and reactors for the production of pharmaceutical nanoparticles: Proof of concept. Chem. Eng. Res. Des. 2009, 87, 543–549. [Google Scholar] [CrossRef]
- Lince, F.; Marchisio, D.L.; Barresi, A.A. A comparative study for nanoparticle production with passive mixers via solvent-displacement: Use of CFD models for optimization and design. Chem. Eng. Process. 2011, 50, 356–368. [Google Scholar] [CrossRef]
- Song, K.H.; Highley, C.B.; Rouff, A.; Burdick, J.A. Complex 3D-Printed Microchannels within Cell-Degradable Hydrogels. Adv. Funct. Mater. 2018, 28, 1801331. [Google Scholar] [CrossRef]
- Daly, A.C.; Pitacco, P.; Nulty, J.; Cunniffe, G.M.; Kelly, D.J. 3D printed microchannel networks to direct vascularisation during endochondral bone repair. Biomaterials 2018, 162, 34–46. [Google Scholar] [CrossRef]
- Goh, G.L.; Agarwala, S.; Yong, W.Y. 3D printing of microfludic sensor for soft robots: A preliminary study in design and fabrication. In Proceedings of the 2nd International Conference on Progress in Additive Manufacturing, Singapore, 16–19 May 2016; pp. 177–181. [Google Scholar] [CrossRef]
- Enders, A.; Siller, I.G.; Urmann, K.; Hoffmann, M.R.; Bahnemann, J. 3D printed microfluidic mixers—A comparative study on mixing unit performances. Small 2019, 15, 1804326. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Fan, W.; Liu, T. Fused deposition modeling 3D printing of polyamide-based composites and its applications. Compos. Commun. 2020, 21, 100413. [Google Scholar] [CrossRef]
- Gupta, V.; Nesterenko, P.; Paull, B. 3D Printing in Chemical Sciences: Applications Across Chemistry; Royal Society of Chemistry: London, UK, 2019. [Google Scholar]
- Mohamed, O.A.; Masood, S.H.; Bhowmik, J.L. Optimization of fused deposition modeling process parameters: A review of current research and future prospects. Adv. Manuf. 2015, 3, 42–53. [Google Scholar] [CrossRef]
- Dragone, V.; Sans, V.; Rosnes, M.H.; Kitson, P.J.; Cronin, L. 3D-printed devices for continuous-flow organic chemistry. Beilstein J. Org. Chem. 2013, 9, 951–959. [Google Scholar] [CrossRef]
- Hou, W.; Bubliauskas, A.; Kitson, P.J.; Francoia, J.-P.; Powell-Davies, H.; Gutierrez, J.M.P.; Frei, P.; Manzano, J.S.; Cronin, L. Automatic generation of 3D-printed reactionware for chemical synthesis digitization using ChemSCAD. ACS Cent. Sci. 2021, 7, 212–218. [Google Scholar] [CrossRef]
- Zalesskiy, S.S.; Kitson, P.J.; Frei, P.; Bubliauskas, A.; Cronin, L. 3D designed and printed chemical generators for on demand reagent synthesis. Nat. Commun. 2019, 10, 5496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Mubarak, S.; Dhamodharan, D.; Divakaran, N.; Wu, L.; Zhang, X. Fabrication of thermoplastic functionally gradient composite parts with anisotropic thermal conductive properties based on multicomponent fused deposition modeling 3D printing. Compos. Commun. 2020, 19, 142–146. [Google Scholar] [CrossRef]
- Li, H.; Mao, P.; Davis, M.; Yu, Z. PEDOT:PSS-polyethylene oxide composites for stretchable and 3D-Printed thermoelectric devices. Compos. Commun. 2021, 23, 100599. [Google Scholar] [CrossRef]
- Barresi, A.A.; Vanni, M.; Fissore, D.; Zelenkova, T. Synthesis and preservation of polymer nanoparticles for pharmaceutical applications. In Handbook of Polymers for Pharmaceutical Technologies, Processing and Applications; Thakur, V.K., Ed.; Scrivener Publishing L.L.C: Salem, MA, USA, 2015; Volume 2, pp. 229–280. [Google Scholar]
- Lannibois, H.; Hasmy, A.; Botet, R.; Chariol, O.; Cabane, B. Surfactant limited aggregation of hydrophobic molecules in water. J. Phys. II 1997, 7, 319–342. [Google Scholar] [CrossRef]
- Badri, W.; Miladi, K.; Robin, S.; Viennet, C.; Nazari, Q.A.; Agusti, G.; Fessi, H.; Elaissari, A. Polycaprolactone based nanoparticles loaded with indomethacin for anti-inflammatory therapy: From preparation to ex vivo study. Pharm. Res. 2017, 34, 1773–1783. [Google Scholar] [CrossRef]
- Croisier, F.; Atanasova, G.; Poumay, Y.; Jerome, C. Polysaccharide-coated PCL nanofibers for wound dressing applications. Adv. Healthc. Mater. 2014, 3, 2032–2039. [Google Scholar] [CrossRef]






| PCL Concentration (wt%) | Particle Size, Discontinuous Process (nm) | Variability (nm) |
|---|---|---|
| 0.01 | 133 ± 3 | 73 ± 9 |
| 0.05 | 180 ± 2 | 90 ± 7 |
| 0.10 | 192 ± 2 | 84 ± 5 |
| 0.25 | 230 ± 5 | 71 ± 10 |
| 0.50 | 243 ± 3 | 94 ± 8 |
| 1.00 | 262 ± 4 | 95 ± 10 |
| PCL Concentration (wt%) | Particle Size Continuous Process (nm) | Variability (nm) |
|---|---|---|
| 0.01 | 138 ± 3 | 62 ± 8 |
| 0.05 | 179 ± 2 | 58 ± 5 |
| 0.10 | 221 ± 8 | 109 ± 19 |
| 0.25 | 256 ± 4 | 102 ± 9 |
| 0.50 | 262 ± 3 | 101 ± 8 |
| Concentration of PCL (wt%) | Particle Size with Acetone (nm) | Particle Size after Evaporation (nm) | Difference in Particle Size (nm) |
|---|---|---|---|
| 0.01 | 155 ± 1 | 119 ± 2 | 36 |
| 0.05 | 208 ± 2 | 183 ± 3 | 25 |
| 0.10 | 236 ± 3 | 206 ± 3 | 30 |
| 0.25 | 271 ± 3 | 252 ± 4 | 19 |
| 0.50 | 318 ± 5 | 299 ± 5 | 19 |
| Configuration | Particle Size (nm) | Variability of the Particle Sizes (nm) |
|---|---|---|
| 0.1% PCL | 236 ± 3 | 88 ± 6 |
| 0.5 mg LAT50 | 215 ± 8 | 77 ± 18 |
| 1 mg LAT50 | 268 ± 4 | 136 ± 12 |
| 2 mg LAT50 | 260 ± 3 | 132 ± 8 |
| 0.5 mg T80 | 293 ± 5 | 165 ± 12 |
| 1 mg T80 | 276 ± 3 | 110 ± 8 |
| 2 mg T80 | 312 ± 4 | 178 ± 10 |
| Configuration | Particle Size (nm) | Variability of the Particle Sizes (nm) | Zeta Potential (mV) |
|---|---|---|---|
| 0.1% PCL | 236 ± 3 | 88 ± 6 | −14 |
| 0.5% LAT50 in aq. | 246 ± 6 | 111 ± 16 | −5 |
| 0.5% T80 in aq. | 279 ± 3 | 141 ± 7 | 8 |
| 0.1% SDS in aq. | 262 ± 2 | 127 ± 4 | −62 |
| 0.1% CTAB in aq. | 252 ± 2 | 128 ± 5 | +41 |
| 0.1% SDS post formulation. | 271 ± 4 | 125 ± 9 | −51 |
| 0.1% CTAB post formulation. | 281 ± 6 | 168 ± 14 | +51 |
| Polymer Concentration (wt%) | Particle Size (nm) | Variability of the Particle Sizes (nm) |
|---|---|---|
| 0.01% | 155 ± 1 | 55 ± 2 |
| 0.05% | 208 ± 2 | 89 ± 6 |
| 0.1% | 246 ± 3 | 88 ± 6 |
| 0.01% + NR | 159 ± 3 | 64 ± 6 |
| 0.05% + NR | 208 ± 2 | 90 ± 6 |
| 0.1% + NR | 219 ± 3 | 86 ± 6 |
| Attempt | Particle Size (nm) | Variability of the Particle Sizes (nm) |
|---|---|---|
| 0.1% PCL T-Micromixer 0.1% PCL T2-Micromixer | 223 ± 3 224 ± 2 | 87 ± 7 80 ± 5 |
| 0.1% PCL Y-Micromixer | 236 ± 3 | 88 ± 6 |
| 0.1% PCL focus geometry | 229 ± 4 | 79 ± 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Göttert, S.; Salomatov, I.; Eder, S.; Seyfang, B.C.; Sotelo, D.C.; Osma, J.F.; Weiss, C.K. Continuous Nanoprecipitation of Polycaprolactone in Additively Manufactured Micromixers. Polymers 2022, 14, 1509. https://doi.org/10.3390/polym14081509
Göttert S, Salomatov I, Eder S, Seyfang BC, Sotelo DC, Osma JF, Weiss CK. Continuous Nanoprecipitation of Polycaprolactone in Additively Manufactured Micromixers. Polymers. 2022; 14(8):1509. https://doi.org/10.3390/polym14081509
Chicago/Turabian StyleGöttert, Simeon, Irina Salomatov, Stephan Eder, Bernhard C. Seyfang, Diana C. Sotelo, Johann F. Osma, and Clemens K. Weiss. 2022. "Continuous Nanoprecipitation of Polycaprolactone in Additively Manufactured Micromixers" Polymers 14, no. 8: 1509. https://doi.org/10.3390/polym14081509
APA StyleGöttert, S., Salomatov, I., Eder, S., Seyfang, B. C., Sotelo, D. C., Osma, J. F., & Weiss, C. K. (2022). Continuous Nanoprecipitation of Polycaprolactone in Additively Manufactured Micromixers. Polymers, 14(8), 1509. https://doi.org/10.3390/polym14081509