Silicalite-1/PDMS Hybrid Membranes on Porous PVDF Supports: Preparation, Structure and Pervaporation Separation of Dichlorobenzene Isomers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
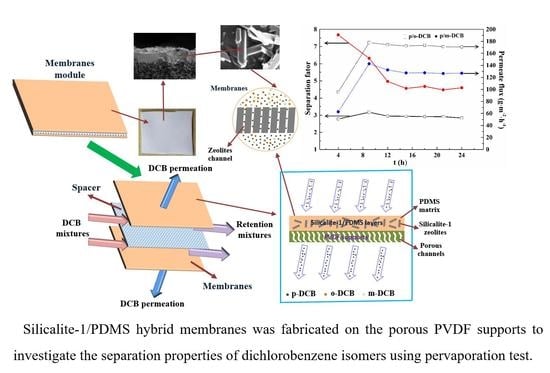
2.2. Preparation of Silicalite-1/PDMS/PVDF Hybrid Membranes
2.3. Characterization
2.4. Pervaporation Evaluation Experiment
3. Results
3.1. Morphology and Structure of Silicalite-1/PDMS/PVDF Hybrid Membranes
3.2. Pervaporation Evaluation Experiment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, R.; Wang, S.; Wang, L. Atmospheric oxidation mechanism of chlorobenzene. Chemosphere 2014, 111, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Netskina, O.V.; Tayban, E.S.; Moiseenko, A.P.; Komova, O.V.; Mukha, S.A.; Simagina, V.I. Removal of 1,2-dichlorobenzene from water emulsion using adsorbent catalysts and its regeneration. J. Hazard. Mater. 2015, 285, 84–93. [Google Scholar] [CrossRef] [PubMed]
- He, Q.H.; Zou, Y.; Wang, P.F.; Dou, X.M. MFI-type zeolite membranes for pervaporation separation of dichlorobenzene isomers. ACS Omega 2021, 6, 8456–8462. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.J.; Li, Y.H.; Luan, Z.K.; Di, Z.C.; Wang, H.Y.; Tian, B.H.; Jia, Z.P. Adsorption of 1,2-dichlorobenzene from water to carbon nanotubes. Chem. Phys. Lett. 2003, 376, 154–158. [Google Scholar] [CrossRef]
- Guo, G.Q.; Long, Y.C. Static equilibrium studies on separation of dichlorobenzene isomers on binder-free hydrophobic adsor-bent of MFI type zeolite. Sep. Purif. Technol. 2001, 24, 507–518. [Google Scholar] [CrossRef]
- He, Q.H.; Zou, Y.; Wang, P.F.; Dou, X.M. Synthesis of silicalite-1 zeolite membranes for vapor-permeation separation of dichlorobenzene isomers. Nanotechnology 2021, 32, 475708. [Google Scholar] [CrossRef]
- McCulloch, B.; Gatter, M.G. Process for Extracting Meta-Dichlorobenzene from Isomer Mixtures with Mixed Alkali Metal Exchanged X Zeolite Adsorbents. U.S. Patent 1989,4996380, 26 February 1991. [Google Scholar]
- Miwa, K.; Nagaoka, Y.; Inoue, T. Process for Separation of Substituted Benzene Isomers. U.S. Patent 1983,4571441, 18 February 1986. [Google Scholar]
- Siegbert, R.; Adolf, S.; Rudolf, S.; Leonhard, U. Separation of m and p-Dichlorobenzene. U.S. Patent 1992,5152875A, 6 October 1992. [Google Scholar]
- Li, Q.; Liu, H.; Wang, B.; Zhang, Y. High purity separation of eutectic mixtures in a continuous moving crystal bed column crystallization. Chem. Eng. Res. Des. 2003, 81, 1373–1378. [Google Scholar] [CrossRef]
- Anonymous. Novel crystallization and separation technique for p-DCB. Filtr. Sep. 2003, 40, 18–19. [Google Scholar] [CrossRef]
- Pies, M.; Rohlk, K.; Lahr, H.; Fiege, H. Process for Isolating m-Dichlorobenzene from Mixtures of Dichlorobenzene Isomers. U.S. Patent 1995,5436377A, 25 July 1995. [Google Scholar]
- Erdem, G.; Leckebusch, M.; Olf, G.; Rinck, K.J.; Zühlke, G. Process for the Separation of Mixtures Containing m- and p-di-chlorobenzene. U.S. Patent 2007,7311807B2, 25 December 2007. [Google Scholar]
- Zhou, Y.X.; Chen, W.; Wang, P.F.; Zhang, Y.M. EMT-type zeolite for deep purification of trace polar-oxygenated compounds from light olefins. Microporous Mesoporous Mater. 2018, 271, 273–283. [Google Scholar] [CrossRef]
- Mihaylov, M.; Ivanova, E.; Chakarova, K.; Kefirov, R.; Kukeva, R.; Stoyanova, R.; Hadjiivanov, K. Purification of hydrogen from CO with Cu/ZSM-5 adsorbents. Molecules 2022, 27, 96. [Google Scholar] [CrossRef]
- Buema, G.; Trifas, L.; Harja, M. Removal of toxic copper ion from aqueous media by adsorption on fly ash-derived zeolites: Kinetic and equilibrium studies. Polymers 2021, 13, 3468. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.F.; Liu, J.Q.; Liu, P.X.; Li, L.B.; Tang, X.; Shang, H.; Li, J.P.; Chen, B.L. K-chabazite zeolite nanocrystal aggregates for highly efficient methane separation. Angew. Chen. Int. Ed. 2022, 61, e202116850. [Google Scholar]
- Johnson, B.A.; Di Iorio, J.R.; Roman-Leshkov, Y. Tailoring distinct reactive environments in lewis acid zeolites for liquid phase catalysis. Acc. Chem. Res. 2021, 2, 1033–1046. [Google Scholar] [CrossRef]
- Li, N.; Xing, X.; Cheng, J.; Zhang, Z.S.; Hao, Z.P. Influence of oxygen and water content on the formation of polychlorinated organic by-products from catalytic degradation of 1,2-dichlorobenzene over a Pd/ZSM-5 catalyst. J. Hazard. Mater. 2021, 403, 123952. [Google Scholar] [CrossRef]
- Pentling, U.; Buysch, H.J.; Puppe, L.; Roehlk, K.; Grosser, R.; Paul, H.I. Process for Separating Mixtures of m- and p-dichlo-robenzene. U.S. Patent 1995,5386067A, 31 January 1995. [Google Scholar]
- Pentling, U.; Buysch, H.J.; Puppe, L. Separation of mixtures of m- and p-dichlorobenzene using pentasil zeolites. Zeolites 1995, 15, 183. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Chen, W.; Wang, P.F.; Zhang, Y.M. Dense and thin 13X membranes on porous-Al2O3 tubes: Preparation, structure and deep purification of oxygenated compounds from gaseous olefin flow. RSC Adv. 2018, 8, 13728–13738. [Google Scholar] [CrossRef] [Green Version]
- Kyotani, T.; Richter, H. Zeolite membrane: From microstructure to separation performance. Membranes 2022, 12, 176. [Google Scholar] [CrossRef]
- Tavolaro, P.; Martino, G.; Ando, S.; Tavolaro, A. Fabrication and evaluation of novel zeolite membranes to control the neoplastic activity and anti-tumoral drug treatments in human breast cancer cells. Part 1: Synthesis and characterization of pure zeolite membranes and mixed matrix membranes for adhesion and growth of cancer cells. Mat. Sci. Eng. C-Mater. 2016, 69, 894–904. [Google Scholar]
- Aloulou, W.; Aloulou, H.; Ben Amar, R. Low-cost composite ultrafiltration membrane made from TiO2 and nanocomposite clay materials over zeolite support for oily wastewater purification and heavy metals removal. Desalin. Water Treat. 2022, 246, 166–173. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.H.; Wang, J.Q.; Raza, W.; Liu, G.R.; Lu, J.M.; Zhang, Y. Microwave synthesis of NaA zeolite membranes on coarse macroporous alpha-Al2O3 tubes for desalination. Microporous Mesoporous Mater. 2020, 306, 110360. [Google Scholar] [CrossRef]
- Ma, J.; Shao, J.; Wang, Z.B.; Yan, Y.S. Preparation of zeolite NaA membranes on macroporous alumina supports by secondary growth of gel layers. Ind. Chem. Eng. Res. 2014, 53, 6121–6130. [Google Scholar] [CrossRef]
- Richard, W.B.; Kaaeid, L. Natural gas processing with membranes: An overview. Ind. Chem. Eng. Res. 2008, 47, 2109–2121. [Google Scholar]
- Li, Y.; Liu, J.; Yang, W. Formation mechanism of microwave synthesized LTA zeolite membranes. J. Membr. Sci. 2006, 281, 646. [Google Scholar] [CrossRef]
- Li, J.; Rong, H.Z.; Chen, C.; Li, Z.Y.; Zuo, J.Y.; Wang, W.J.; Liu, X.J.; Guan, Y.X.; Yang, X.; Liu, Y.S.; et al. Synthesis optimization of SSZ-13 zeolite membranes by dual templates for N2/NO2 separation. Chem. Res. Chin. Univ. 2022, 38, 250–256. [Google Scholar] [CrossRef]
- Hayakawa, E.; Himeno, S. Preparation of Al-Containing ZSM-58 zeolite membranes using rapid thermal processing for CO2/CH4 mixture separation. Membranes 2021, 11, 623. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, R.; Shao, H.; Zhong, J.; Ren, X.; Zhou, Z. Preparation of heteroatom isomorphously substituted MEL zeolite membranes for pervaporation separation of dimethylformamide/water mixtures. Karean J. Chem. Eng. 2021, 38, 2150–2156. [Google Scholar] [CrossRef]
- Banihashemi, F.; Meng, L.; Babaluo, A.A.; Lin, J.Y.S. Xylene vapor permeation in MFI zeolite membranes made by templated and template-free secondary growth of randomly oriented seeds: Effects of xylene activity and microstructure. Ind. Chem. Eng. Res. 2018, 57, 16059–16068. [Google Scholar] [CrossRef]
- Xia, D.Y.; Peng, L.; Wu, Z.Q.; Wang, L.Z.; Jia, Y.M.; Zhang, C.; Gu, X.H. Two-stage varying-temperature synthesis of MFI zeolite membrane and their separation performance for xylene isomers. Chem. J. Chin. Univ. 2020, 41, 2813–2821. [Google Scholar]
- Sakai, M.; Kaneko, T.; Sasaki, Y.; Sekigawa, M.; Matsukata, M. Formation process of columnar grown (101)-oriented silicalite-1 membrane and its separation property for xylene isomer. Crystals 2020, 10, 949. [Google Scholar] [CrossRef]
- Sakai, M.; Sasaki, Y.; Kaneko, T.; Matsukata, M. Contribution of pore-connectivity to permeation performance of silicalite-1 membrane; part I, pore volume and effective pore size. Membranes 2021, 11, 382. [Google Scholar] [CrossRef]
- Han, S.C.; Liu, P.; Ma, Y.; Wu, Q.M.; Meng, X.J.; Xiao, F.S. Calcination-free fabrication of highly b-oriented silicalite-1 zeolite films by secondary growth in the absence of organic structure directing agents. Ind. Eng. Chem. Res. 2021, 60, 7167–7173. [Google Scholar] [CrossRef]
- Zhuang, X.; Chen, X.; Su, Y.; Luo, J.; Cao, W.; Wan, Y. Improved performance of PDMS/silicalite-1 pervaporation membranes via designing new silicalite-1 particles. J. Membr. Sci. 2015, 493, 37–45. [Google Scholar] [CrossRef]
- Liu, G.P.; Xiangli, F.J.; Wei, W.; Liu, S.N.; Jin, W.Q. Improved performance of PDMS/ceramic composite pervaporation mem-branes by ZSM-5 homogeneously dispersed in PDMS via a surface graft/coating approach. Chem. Eng. J. 2011, 174, 495–503. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.X.; Zhan, X.; Fang, M.Q.; Wang, T.; Li, J.D. Preparation and characterization of ZSM-5/PDMS hybrid per-vaporation membranes: Laboratory results and pilot-scale performance. Sep. Purif. Technol. 2015, 150, 257–267. [Google Scholar] [CrossRef]
- Nigiz, F.U.; Ozkoc, G.; Hilmioglu, N.D. A study on the separation performance of zeolite filled thin film composite poly(dimethyl siloxane) membrane. Mater. Des. 2015, 88, 942–949. [Google Scholar] [CrossRef]
- Casado-Coterillo, C.; Fernandez-Barquin, A.; Valencia, S.; Irabien, A. Estimating CO2/N2 Permselectivity through Si/Al=5 small-pore zeolites/PTMSP mixed matrix membranes: Influence of temperature and topology. Membranes 2018, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Barquín, A.; Casado-Coterillo, C.; Palomino, M.; Valencia, S.; Irabien, A. Permselectivity improvement in mem-branes for CO2/N2 separation. Sep. Purif. Technol. 2016, 157, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Asghari, M.; Mosadegh, M.; Harami, H.R. Supported PEBA-zeolite 13X nano-composite membranes for gas separation: Preparation, characterization and molecular dynamics simulation. Chem. Eng. Sci. 2018, 187, 67–78. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, M.Y.; Feng, X.S.; Wang, X.D.; Huang, W. Ethylene/propylene separation using mixed matrix membranes of poly (ether block amide)/nano-zeolite (NaY or NaA). Korean J. Chem. Eng. 2021, 38, 576–586. [Google Scholar] [CrossRef]
- Mosadegh, M.; Amirkhani, F.; Harami, H.R.; Asghari, M.; Parnian, M.J. Effect of nafion and APTEOS functionalization on mixed gas separation of PEBA-FAU membranes: Experimental study and MD and GCMC simulations. Sep. Purif. Technol. 2020, 247, 116981. [Google Scholar] [CrossRef]
- Nigiz, F.U.; Veli, S.; Hilmioglu, N.D. Deep purification of seawater using a novel zeolite 3A incorporated polyether-block-amide composite membrane. Sep. Purif. Technol. 2018, 188, 90–97. [Google Scholar] [CrossRef]
- Kursun, F. Application of PVA-b-NaY zeolite mixture membranes in pervaporation method. J. Mol. Struct. 2019, 1201, 127170. [Google Scholar] [CrossRef]
- Achari, D.D.; Hegde, S.N.; Pattanashetti, N.A.; Kamble, R.R.; Kariduraganavar, M.Y. Development of zeolite-A incorporated PVA/CS nanofibrous composite membranes using the electrospinning technique for pervaporation dehydration of water/tert-butanol. New J. Chem. 2021, 45, 3981–3996. [Google Scholar] [CrossRef]
- Viboonratanasri, D.; Thongdee, P.; Prajuabsuk, M.; Pungpo, P.; Vayachuta, L.; Prompinit, P. Precisely controlled delivery of plant hormone using poly(vinyl alcohol)/zeolite A hydrofilm composite. Polym. Eng. Sci. 2021, 61, 2172–2182. [Google Scholar] [CrossRef]
- Vane, L.M.; Namboodiri, V.V.; Bowen, T.C. Hydrophobic zeolite-silicone rubber mixed matrix membranes for ethanol-water separation: Effect of zeolite and silicone component selection on pervaporation performance. J. Membr. Sci. 2008, 308, 230–241. [Google Scholar] [CrossRef]
- Tantekin-Ersolmaz, S.B.; Senorkyan, L.; Kalaonra, N.; Tatlier, M.; Erdem-Senatalar, A. n-Pentane/i-pentane separation by using zeolite-PDMS mixed matrix membranes. J. Membrane. Sci. 2001, 189, 59–67. [Google Scholar] [CrossRef]
- Gou, Y.H.; Xiao, L.; Yang, Y.T.; Guo, X.H.; Zhang, F.M.; Zhu, W.D.; Xiao, Q. Incorporation of open-pore MFI zeolite nanosheets in polydimethylsiloxane (PDMS) to isomer-selective mixed matrix membranes. Microporous Mesoporous Mater. 2021, 315, 110930. [Google Scholar] [CrossRef]
- Xue, C.; Du, G.-Q.; Chen, L.-J.; Ren, J.-G.; Bai, F.-W. Evaluation of asymmetric polydimethylsiloxane-polyvinylidene fluoride composite membrane and incorporated with acetone-butanol-ethanol fermentation for butanol recovery. J. Biotechnol. 2014, 188, 158–165. [Google Scholar] [CrossRef]
- Durdakova, T.M.; Hovorka, S.; Hrdlicka, Z.; Vopicka, O. Comparison of pervaporation and perstraction for the separation of p-xylene/m-xylene mixtures using PDMS and CTA membranes. Sep. Purif. Technol. 2021, 274, 118986. [Google Scholar] [CrossRef]
- Wang, N.; Liu, J.; Li, J.; Gao, J.; Ji, S.; Li, J.R. Tuning properties of silicalite-1 for enhanced ethanol/water pervaporation separation in its PDMS hybrid membrane. Microporous Mesoporous Mater. 2015, 201, 35–42. [Google Scholar] [CrossRef]
- Xue, G.P.; Shi, B.L. Performance of various Si/Al ratios of ZSM-5-filled polydimethylsiloxane/polyethersulfone membrane in butanol recovery by pervaporation. Adv. Polym. Tech. 2018, 37, 3095–3105. [Google Scholar] [CrossRef]
- Pattabhi Ramaiah, K.; Satyasri, D.; Sridhar, S.; Krishnaiah, A. Removal of hazardous chlorinated VOCs from aqueous solutions using novel ZSM-5 loaded PDMS/PVDF composite membrane consisting of three hydrophobic layers. J. Hazard. Mater. 2013, 261, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Tan, H.F.; Li, D.M.; Jin, Y. Pervaporation of aqueous solution of acetaldehyde through ZSM-5 filled PDMS composite membrane. Chin. J. Chem. Eng. 2012, 20, 625–632. [Google Scholar] [CrossRef]
- Banihashemi, F.; Pakizeh, M.; Ahmadpour, A. CO2 separation using PDMS/ZSM-5 zeolite composite membrane. Sep. Purif. Technol. 2011, 79, 293–302. [Google Scholar] [CrossRef]
- Ji, L.Y.; Shi, B.L.; Wang, L.L. Pervaporation separation of ethanol/water mixture using modified zeolite filled PDMS membranes. J. Appl. Polym. Sci. 2015, 132, 41897. [Google Scholar] [CrossRef]
- Han, X.L.; Zhang, X.M.; Ma, X.X.; Li, J.D. Modified ZSM-5/polydimethylsiloxane mixed matrix membranes for ethanol/water separation via pervaporation. Polym. Compos. 2016, 37, 1282–1291. [Google Scholar] [CrossRef]
- Sun, W.; Wang, X.; Yang, J.; Lu, J.; Han, H.; Zhang, Y.; Wang, J. Pervaporation separation of acetic acid–water mixtures through Sn-substituted ZSM-5 zeolite membranes. J. Membr. Sci. 2009, 335, 83–88. [Google Scholar] [CrossRef]
- Jacobs, P.A.; Beyer, H.K.; Valyon, J. Properties of the end members in the Pentasil-family of zeolites: Characterization as adsorbents. Zeolites 1981, 1, 161–168. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Aly, H.M.; El-Shahat, M.F.; Ibrahim, I.A. Effect of the silica sources on the crystallinity of nanosized ZSM-5 zeolite. Microporous Mesoporous Mater. 2005, 79, 7–12. [Google Scholar] [CrossRef]
- Xue, T.; Wang, Y.M.; He, M.-Y. Synthesis of ultra-high-silica ZSM-5 zeolites with tunable crystal sizes. Solid Stade Sci. 2012, 14, 409–418. [Google Scholar] [CrossRef]
- Coppens, M.O.; Sun, J.H.; Maschmeyer, T. Synthesis of hierarchical porous silicas with a controlled pore size distribution at various length scales. Catal. Today 2001, 69, 331–335. [Google Scholar] [CrossRef]
- Hua, J.; Han, Y. One-step preparation of zeolite silicalite-1 microspheres with adjustable microporosity. Chem. Mater. 2009, 21, 2344–2348. [Google Scholar] [CrossRef]
- Zhao, R.H.; Chen, J.Y.; Liu, J.M.; Fan, J.; Du, J.P. Morphologies-controlling synthesis of silicalite-1 and its adsorption property. Mater. Lett. 2015, 139, 494–497. [Google Scholar] [CrossRef]
- Jessie Lue, S.J.; Chien, C.F.; Mahesh, K.P.O. Pervaporative concentration of ethanol–water mixtures using heterogeneous polydimethylsiloxane (PDMS) mixed matrix membranes. J. Membr. Sci. 2011, 384, 17–26. [Google Scholar]
- Vera-Graziano, R.; Hernandez-Sanchez, F.; Cauich-Rodriguez, J.V. Study of crosslinking density in polydimethylsiloxane networks by DSC. J. Appl. Polym. Sci. 2003, 55, 1317–1327. [Google Scholar] [CrossRef]
- Deng, X.B.; Liu, B.L.; Cao, S.S.; Luo, R.; Chen, H.L. A novel approach for the preparation of PMMA–PDMS core–shell particles with PDMS in the shell. Appl. Surf. Sci. 2007, 253, 4823. [Google Scholar] [CrossRef]
- Liang, L.; Ruckenstein, E. Pervaporation of ethanol-water mixtures through polydimethylsiloxane-polystyrene interpenetrating polymer network supported membranes. J. Membr. Sci. 1996, 114, 227–234. [Google Scholar] [CrossRef]
- Hijon, N.; Manzano, M.; Salinas, A.J.; Vallet-Regi, M. Bioactive CaO–SiO2–PDMS coatings on Ti6Al4V substrates. Chem. Mater. 2005, 17, 1591–1596. [Google Scholar] [CrossRef]
- Xiangli, F.; Chen, Y.; Jin, W.; Xu, N. Polydimethylsiloxane/ceramic composite membrane with high flux for pervaporation of ethanol–water mixtures. Ind. Eng. Chem. Res. 2007, 46, 2224–2230. [Google Scholar] [CrossRef]
- Xia, Z.; Ding, L.J.; Qi, H.J.; Xian, C.C. Pervaporation properties of PDMS membranes cured with different cross-linking reagents for ethanol concentration from aqueous solutions. Chin. J. Polym. Sci. 2009, 27, 533–542. [Google Scholar]
- Han, X.L.; Wang, L.; Li, J.D.; Zhan, X.; Chen, J.; Yang, J.C. Tuning the hydrophobicity of ZSM-5 zeolites by surface silanization using alkyltrichlorosilane. Appl. Surf. Sci. 2011, 257, 9525–9531. [Google Scholar] [CrossRef]
- Kordatos, K.; Gavela, S.; Ntziouni, A.; Pistiolas, K.; Kyritsi, A.; Kasselouri-Rigopoulou, V. Synthesis of highly siliceous ZSM-5 zeolite using silica from rice husk ash. Microporous Mesoporous Mater. 2008, 115, 189–196. [Google Scholar] [CrossRef]
- Mei, E.; Bardo, A.M.; Collinson, M.M.; Higgins, D.A. Single-molecule studies of sol–gel-derived silicate films. Microenvironments and film-drying conditions. J. Phys. Chem. B 2000, 104, 9973–9980. [Google Scholar] [CrossRef]
- Jansen, J.C.; van der Gaag, F.J.; van Bekkum, H. Identification of ZSM-type and other 5-ring containing zeolites by i.r. spectroscopy. Zeolites 1984, 4, 369–372. [Google Scholar] [CrossRef]
- Donato, L.; Garofalo, A.; Drioli, E.; Alharbi, O.; Aljlil, S.A.; Criscuoli, A.; Algieri, C. Improved performance of vacuum membrane distillation in desalination with zeolite membranes. Sep. Purif. Technol. 2020, 237, 116376. [Google Scholar] [CrossRef]














| Zeolites Sample | SBET (m2/g) | Smic (m2/g) | Sext (m2/g) | Vmic (cm3/g) | Vmeso (cm3/g) | Daver (nm) | Vtotal (cm3/g) |
|---|---|---|---|---|---|---|---|
| Silicalite-1 | 356.1 | 296.4 | 59.7 | 0.171 | 0.038 | 2.31 | 0.209 |
| ZSM−5 (SiO2/Al2O3 = 300) | 356.0 | 186.1 | 169.9 | 0.090 | 0.145 | 2.68 | 0.235 |
| p/o−DCB Mixtures | p/m−DCB Mixtures | |||||
|---|---|---|---|---|---|---|
| JN,p/o−DCB | JN,p−DCB | JN,o−DCB | JN,p/m−DCB | JN,p−DCB | JN,m−DCB | |
| Silicalite-1/α−Al2O3 membranes(μm∙kg∙m−2∙h−1) | 2.14 | 1.49 | 0.65 | 1.75 | 1.33 | 0.42 |
| Sillicate−1/PDMS/PVDF hybrid membranes (μm∙kg∙m−2∙h−1) | 2.99 | 2.21 | 0.78 | 2.22 | 1.83 | 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Chen, W.; Wang, P.; Dou, X. Silicalite-1/PDMS Hybrid Membranes on Porous PVDF Supports: Preparation, Structure and Pervaporation Separation of Dichlorobenzene Isomers. Polymers 2022, 14, 1680. https://doi.org/10.3390/polym14091680
He Q, Chen W, Wang P, Dou X. Silicalite-1/PDMS Hybrid Membranes on Porous PVDF Supports: Preparation, Structure and Pervaporation Separation of Dichlorobenzene Isomers. Polymers. 2022; 14(9):1680. https://doi.org/10.3390/polym14091680
Chicago/Turabian StyleHe, Qiuping, Wei Chen, Pengfei Wang, and Xiaoming Dou. 2022. "Silicalite-1/PDMS Hybrid Membranes on Porous PVDF Supports: Preparation, Structure and Pervaporation Separation of Dichlorobenzene Isomers" Polymers 14, no. 9: 1680. https://doi.org/10.3390/polym14091680
APA StyleHe, Q., Chen, W., Wang, P., & Dou, X. (2022). Silicalite-1/PDMS Hybrid Membranes on Porous PVDF Supports: Preparation, Structure and Pervaporation Separation of Dichlorobenzene Isomers. Polymers, 14(9), 1680. https://doi.org/10.3390/polym14091680