1. Introduction
The theoretical calculation of bonding energy in the polymer is important. It helps to screen the formulations to avoid many unnecessary lab reactions; eventually, it can save the chemicals and time. Thus, it is highly beneficial for the environment by using only necessary chemicals and is cost-effective by arranging the required chemicals only. The widely used theoretical calculation method of bonding is the density functional theory (DFT) method. Especially, the hydrogen bond energy calculation by the DFT method has been proved to be an efficient tool. The value of binding energy is an indication of the strength of hydrogen bond of functional groups. The binding energy value changes with the nature and content of the monomers. A higher value indicates more hydrogen bonding between certain functional groups in the polymer. This will ultimately improve the properties. Thus, this calculation will help to choose the appropriate monomers and their contents to synthesize polymers in a laboratory [
1,
2,
3,
4].
Polyurethane (PU) is a multifunctional polymer. Compared to other traditional polymers, PU usually possesses superior properties. Nowadays, PU is widely being used in many different areas especially in coating, adhesive, and biomaterials [
5,
6,
7,
8]. By adopting new techniques, the PU material is extended its new areas of applications, especially for harsh conditions. Urethane (NHCOO) and urea (NHCONH) are two main functional groups in PU. The urethane group is formed by reacting hydroxyl (OH) and isocyanate (NCO) groups; and the urea group by reacting amine (NH
2) and NCO groups. Different polyether polyol, polyester polyol, acrylate polyol, and siloxane polyol with their different molecular weight acts for -OH group, whereas aliphatic and aromatic isocyanates act for the NCO group. These monomers not only form the urethane-urea groups but also contribute to hydrogen bond (HB) formation, providing their available electronegative atoms to participants. The newly formed urethane and urea groups also participate in hydrogen bonding. In our previous study [
9], it had been shown that the hydrogen bond density, which is an indication of the strength of HB, was changed by changing monomers and their contents. The HB quantity increased up to a certain EDA content [
9]. In another study, we showed that the HB increased with increasing the DMPA content [
10]. We also showed that the HB increased when lignin was used along with EDA as a chain extender [
11]. It had been shown that the polymer properties were hugely affected by their HB. The adhesive strength, hydrophobicity, mechanical strength, and corrosion resistance all increased with increasing HB [
8,
9,
10,
11]. Self-healing of materials was also improved by increasing HB [
12]. Rahman et al. [
12] used a certain level of monomer to have a supramolecular structure to increase the HB, which ultimately improved the self-healing nature of materials. However, excessive HB can derail the properties [
9]. It is important to maintain the proper balance of monomers to keep the HB at a certain level.
PU is widely being used for adhesion purposes in the textile industry [
13]. Unfortunately, commercial PU adhesives mostly contain toxic organic solvents, which are emitted during the synthesis and drying stages [
5]. Thus, it threatens the environment as well as human health. Many countries restricted these organic solvents, used to save human health and the environment. Due to this limitation, the trends of using organic coatings slowly changed from solvent-based to water-based/waterborne polyurethane (WBPU) adhesives. Unfortunately, the water-based PU adhesive has comparatively less adhesive strength. Significant efforts were found by many researchers to improve the adhesive strength of WBPU. They used different DMPA contents [
10], polyols [
14], hardeners [
10], crosslinkers [
15] and nanoparticles [
16]. The adhesive strength also varied with pressed temperature during the bonding of two nylon fabrics [
10]. The adhesive strength was improved by maintaining a proper stoichiometric ratio of monomers [
10]. The main challenge appears at moderately high temperatures and under water conditions. The adhesive strength usually falls in both cases [
10,
15]. Scientists are still working to improve the WBPU adhesion at moderately high temperatures and wet conditions.
There are monomers which are a mixture of isomers. The effect of isomers on properties is mostly overlooked in WBPU materials. The widely used monomer H
12MDI is a mixture of isomers (cis-cis, trans-trans, cis-trans). Saralegi et al. [
16] separated the isomers of H
12MDI. The mechanical strength and thermal stability are hugely affected due to their different isomer structures. Catechol (CC) and hydroquinone (HQ) are isomers, with the same chemical formula as C
6H
4(OH)
2 and a molecular weight of 110.1 g/mol, of 1, 2-dihydroxybenzene (DHB) and 1, 4-dihydroxybenzene, respectively. The DHB is used in polymeric materials to improve their mechanical strength, thermal stability, and hydrophobicity. The DHB is also used in PU. Ren et al. [
17] used a CC-based compound in PU. The highest level of thermal stability, mechanical strength, and adhesive strength were recorded at the ratio 25:4 of NCO and CC. They proved that the proper CC content can only improve the properties at a significant level. Huang et al. [
18] used HQ in PU to improve the mechanical and thermal properties. It is hard to find any report comparing the DHB isomer effect on WBPU properties. This scenario makes us interested in investigating the effect of hydrogen bonds (due to the different positions of -OH groups) between CC/HQ and urethane/urea groups on the properties. Such an investigation can further help to assess the effect of similar types of other isomeric monomer structures on polymer properties. We counted both theoretically and experimentally hydrogen bonds and their ultimate effect on WBPU properties. To the best of our knowledge, such a type of comparisons has not been considered yet, especially in PU materials. In this study, two series of WBPU dispersions, using defined CC and HQ contents, were prepared. The dispersion sacrificed their shelf life when the CC and HQ contents were above 2.0 wt%. Thus, the maximum used CC and HQ content was 2.0 wt% during the WBPU dispersions. The CC and HQ attachments in PU were theoretically analyzed by DFT calculation. All synthesized dispersions used an adhesive material to bond nylon fabrics. The adhesive strength was evaluated at a moderately high temperature. The hydrophobicity and thermal stability properties were also evaluated.
3. Results and Discussion
All dispersions were prepared by an in-situ polymerization method following our previous report [
10]. WBPU polymer was identified by FT-IR spectroscopy. Typical FT-IR spectra is given in
Figure 2. Almost a similar type of spectra and pattern were recorded for all the polymers. A few typical peaks at 2919 (-C-H), 1624 (-C=C), 1247 (-C-N), 1144 (-C-O-C), 1001 (-C=C) cm
−1 appeared almost at the same position for all the films. The peaks at 3430 and 1710 cm
−1 imply NH and CO groups, respectively. The peaks of NH and CO groups, which are identical for urethane and urea confirmed PU polymer was prepared properly [
10]. The peak at 3430 cm
−1 was broadened after DHB addition. The wide peak is attributed to the presence of OH groups which appeared for DHB. The observation that both peaks at 3430 and 1710 cm
−1 shifted to lower values with addition of DHB also implied hydrogen bonding by the addition of DHB. A much lower shifted value with higher DHB content was also recorded (not shown), which confirmed a higher level of HB. The large number of hydrogen bonding can be ascribed by available OH groups, which can easily participate in this bonding. Surprisingly, it was also found that a much lower shifting value was recorded for CC than HQ at the same composition. The difference is clearly seen in higher contents of CC and HQ (not shown). This means that CC involved more HB. The -OH position in CC might make a difference and have greater hydrogen bonding in WBPU.
To confirm the hydrogen bonding of CC and HQ in WBPU, the DFT method was employed. In WBPU (see
Figure 1), urethane and urea groups are two active sites where analytes (catechol and hydroquinone) can bind. Firstly, a catechol has been placed 3.0 Å above the urethane group in WBPU and optimized with the above-mentioned level of theory. The three different approaches (see
Figure 3) have been discussed below for WBPU-catechol complex formation through H-bonds. In the first approach (CC1), the –OH
catechol forms a H-bond with the oxygen atom of the carbonyl group in urethane with a bond length 1.76 Å. In the second approach (CC2), two –OH
catechol form two H-bonds with oxygen atom and nitrogen atom of carbonyl and amine group in urethane with 1.80 Å and 2.07 Å bond lengths, respectively. In the third approach (CC3), one –OH
catechol forms a hydrogen bond with the oxygen atom of ether group in urethane with 1.86 Å bond length. Among the three approaches, CC1 exhibits the most significant complex formation energy (ΔE
BE,cp) −8.81 kcal/mol between WBPU and catechol. In the CC2 and CC3 approaches, the energies are −6.23 kcal/mol and −3.66 kcal/mol, which are 2.42 kcal/mol and 4.75 kcal/mol less than that of CC1, respectively. Later on, the catechol is placed on urea linkage and forms H- bonds through two approaches (CU1 and CU2) (
Figure 4). In CU1, one -OH group of catechol forms a H-bond with the oxygen atom of urea with bond length 1.70 Å. Here, the calculated
ΔEBE is −11.84 kcal/mol, which is 3.41 kcal/mol higher than in the CC1 approach. In CU2, two –OH groups form two H-bonds with two linked urea groups. One -OH group forms an H-bond with the oxygen atom of one urea with bond length 1.67 Å and another –OH group forms H-bond with the hydrogen atom of –NH group of urea with bond length 2.21 Å. In the CU2 approach, the calculated binding energy is −12.36 kcal/mol, which is 0.52 kcal/mol higher than the CU1 approach.
Similarly, hydroquinone was placed on the urethane group at a 3.0 Å distance. Here, hydroquinone followed two approaches (HC1 and HN1) to interact with the urethane group (
Figure 5). In the HC1 approach, -OH
hydroquinone forms two H-bonds with urethane, one with the oxygen atom of carbonyl group and another with the hydrogen atom of -NH group in urethane with bond lengths 1.78 Å and 2.22 Å, respectively. The calculated
ΔEBE of this approach is −9.56 kcal/mol, which is 2.80 kcal/mol lower than the CU2 approach of catechol. In the HN1 approach, hydrogen atom of -OH
hydroquinone forms a hydrogen bond with the nitrogen atom of –NH in the urethane group with a bond length 2.16 Å. Here,
ΔEBE is 6.66 kcal/mol lower than the HC1 approach. When hydroquinone is placed over urea groups, HU1 and HU2, two approaches are observed (
Figure 5). In HU1, -OH
hydroquinone forms H-bond with oxygen atom of carbonyl group of urea with bond length 1.75 Å. Here, the calculated
ΔEBE is −10.29 kcal/mol which is 0.73 kcal/mol higher than that of the HC1 approach. Nevertheless, it is 1.22 kcal/mol lower than that of the CU2 approach. In the HU2 approach, -OH
hydroquinone forms two hydrogen bonds with two ureas, one with a carbonyl group of one urea and another with the –NH group of another urea with bond lengths 1.75 Å and 2.27 Å, respectively. The highest complex formation energy, −11.51 kcal/mol, has been obtained in this approach. This value is 1.10 kcal/mol lower than that of the CU2 approach. It implies that catechol can bind with WBPU through a CU2 approach more strongly than hydroquinone. All complex formation energies are shown in
Table 2.
The natural bond orbital (NBO) analysis has been performed to estimate the charge transfer by catechol and hydroquinone and to confirm the H-bond formation during complex formation with WBPU. In each approach, catechol and hydroquinone detract charges from WBPU, as shown in
Table 2. In the CU2 approach, catechol detracts the most considerable amount of charge 0.038 e
− from WPU. On the other hand, in the HU2 approach, hydroquinone detracts 0.031 e
− the amount of charge from WBPU which is 0.007 e
− less than that of the CU2 approach. It manifests in a stronger binding between WBPU and catechol than that of WBPU and hydroquinone. Besides this, to analyze the interaction for intermolecular H-bond formation, NBO analysis has been considered. This technique can provide the most possible natural Lewis structure picture of orbitals. In the complex, an H-bond forms due to intermolecular interactions which arise from electron density transfer from filled lone pair electrons (
n) of the “Lewis base” into the unfilled antibonding (σ*) of the “Lewis acid”. The strength of interactions, E
2, is estimated by second-order perturbation theory [
21]. In WBPU-catechole complex, NBO analysis evidence the formation of two H-bonds, of which one is n1(O54)→σ*(O138-H144) with the highest stabilization energy 11.68 kcal/mol and another is n2(O137)→σ*(N21-H46) with stabilization energy 0.80 kcal/mol. Between two H-bonds, the C-O---H-O bond is more stable than N-H---O-H. To find the effect of H-bond on the two nearest bonds C17-O54 in WBPU and O138-H144 in catechol, we have analyzed Wiberg bond index (WBI), which ensures the bond strength relative to the overlap population [
4]. The WBIs of C17-O54 and O138-H144 in isolate WBPU and catechol are 1.5705 and 0.7118, respectively. After H-bond formation, the WBIs of C17-O54 and O138-H144 decrease to 1.4614 and 0.5934, respectively. A new H-bond formation between oxygen and hydrogen of O54 and H144 reduces the strength of the two bonds. Similarly, for the second H-bond between H46 and O137, the WBIs of N21-H46 and O137-H148 decrease from 0.7881 and 0.6995 to 0.7589 and 0.5934, respectively.
In the WBPU-hydroquinone complex, NBO analysis evidences the formation of two H-bonds, C17-O54—H144-O140 and N21-H46—O140-H144. The H-bond C17-O54—H144-O140, is observed through n2(O54)→σ*(O140-H144) charge transfer with the highest stabilization energy of 12.16 kcal/mol. Another H-bond N21-H46—O140-H144 is formed through n1(O140)→σ*(N21-H46) charge transfer with stabilization energy 3.53 kcal/mol. Between the two H-bonds, the C17-O54—H144-O140 bond is more stable than N21-H46---O140-H144 by 8.63 kcal/mol. The WBIs of O137-H143 and O140-H144 is 0.7275. After H-bond formation, the WBIs of C17-O54, N21-H46 and O140-H144 decrease to 1.4718, 0.7588 and 0.6231, respectively. Therefore, two newly formed H-bonds decrease the strength of the three bonds. However, in this case, the O137-H143 bond does not take part in any H-bond formation and its WBI does not change.
For further evidence of the formation of WBPU-catechol and WBPU-hydroquinone complexes through H-bonds, we calculated UV-vis spectra of isolated WBPU and WBPU-X complexes using TD-DFT with the above-mentioned level of theory presented in
Figure 6. The estimated
λmax of isolated WBPU is found at 195 nm, which corresponds to the π→π* transition. Two WBPU-X complexes found through CU2 and HU2 approaches were considered for UV-vis spectra calculation because the largest complex formation binding energies for WBPU-catechol and WBPU-hydroquinone have been obtained through these two approaches. For the WBPU-catechol complex, the
λmax is found at 211 nm with a weak band at 252 nm. For WBPU-hydroquinone, the
λmax appears at 223 nm with a weak band at 272 nm. In both WBPU-catechol and WBPU-hydroquinone complexes, the
λmax are red-shifted by 16 nm and 28 nm compared to isolated WBPU. This redshifting of
λmax and newly appeared weak bands confirm the formation of WBPU-catechol and WBPU-hydroquinone complexes.
Conventional WBPU coatings are mainly hydrophilic [
10]. As the WBPU-CC materials have enriched hydrogen bonds, this polymer surface might have less interaction with water or moisture. At the same time, the WBPU-HQ samples might have more interactions. The overall interaction is usually reflected in their hydrophilicity/hydrophobicity. A greater interaction is reflected by showing their hydrophilicity, or conversely showing their hydrophobicity. The hydrophilicity/hydrophobicity was checked by the water contact angle test (see
Table 3). The value increased with increasing CC and HQ content. In WBPU-CC films, the initial value was at 68° (0.5 wt% CC), which increased to 82° with 2.0 wt% CC content. Meanwhile, the WBPU-HQ films also showed an almost similar extent of increase (changed from 67° to 74°). Although the value increased in both cases, the CC films showed higher values with the same CC or HQ content. This indicates a different dynamic of surface in the presence of CC or HQ due to the hydrogen bond. This also clearly implies the occurrence of orientation/dynamics of surface groups with CC and HQ content. Interestingly, WBPU-CC displayed hydrophobic properties, whereas WBPU-HQ exhibited less hydrophobicity. This is attributed to the increased H-bonding in WBPU-CC, which may not allow polar water molecules to adhere to the surface. The more exposed H-bonding atoms in WBPU-HQ will lead to more interactions with water and thus reduce the contact angle. The maximum shifted value was found at 82°for WBPU-CC-200 (2.0 wt% CC).
The melting temperature (T
m) of all films was evaluated by DSC. The T
m values and DSC curves are summarized in
Table 3 and
Figure 7. The T
m of WBPU, which was free from CC or HQ, appeared at 19.61 °C, whereas the T
m of WBPU-CC and WBPU-HQ films were recorded above 19.61 °C. The T
m shifted to higher values gradually with increasing either CC or HQ content. Usually, a clear higher shifting value of T
m is recorded when there is a huge difference in the hydrogen bonding of polymers [
10]. This clearly indicates that the WBPU-CC and WBPU-HQ possess more hydrogen bonding with the addition of either CC or HQ. The difference was more prominent with higher CC/HQ content. With the addition of CC, the T
m shifted 0.50 °C and 3.83 °C with 0.50 wt% and 2.0 wt%, respectively. At the same time, comparing all the polymers between WBPU-CC and WBPU-HQ, the T
m shifting was always higher for WBPU-CC than WBPU-HQ with a fixed CC and HQ content. The maximum shifted values of 3.83 °C and 1.87 °C were recorded with 2.00 wt% CC and HQ addition, respectively. These findings lead to the conclusion that hydrogen bonding worked here as dictating factor and it helped to have materially different properties.
The TGA was applied to evaluate the effect of hydrogen bonds on the thermal stabilities of films. The typical TGA thermographs are shown in
Figure 8. All the graphs are showing similar trends, indicating that the addition of CC or HQ in WBPU has no interference with their degradation properties. It can clearly be seen from the graphs that all of the films showed two steps of degradation. The first step was slower degradation, and the second step was faster degradation. The degradation criteria confirm that the polymers are mainly stable up to their first degradation step. The degradation temperature is enhanced by the addition of either CC or HQ. It can also be seen that the degradation temperature is always higher for CC than for HG. This confirmed that the CC-based WBPU had a better thermal stability. This can be ascribed to hydrogen bonds. As the bond energies of CC-based films are higher, more energy is needed for degradation. Thus, the thermal stability automatically improved for CC-based films.
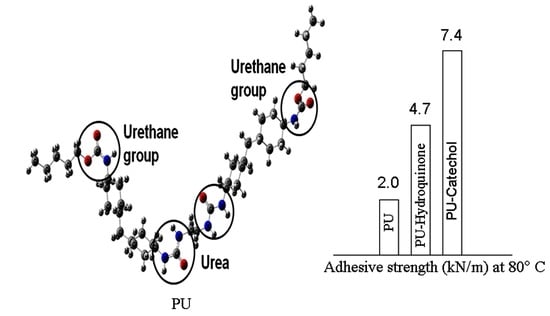
All the dispersions were used to bonded nylon fabrics. The WBPU materials act as an adhesive material. The adhesive strength was measured at room temperature and a defined moderate–high temperature at 80 °C. The respective adhesive strength values are summarized in
Table 3. The adhesive strength increased with the addition of either CC or HQ. The adhesive strength continued to increase with the increase of CC or HQ content. However, the increasing rate was not the same with CC or HQ addition. As expected, the CC series showed a higher adhesive strength compared to those of the HQ series. The hydrogen bond, which was higher in the CC series, made the difference for higher adhesive strength. As hydrogen bond energy was higher in CC-based adhesive materials, the mechanical interlocking was stronger in CC-based samples. Obviously, more energy was needed to de-bond it. This was reflected in the higher adhesive strength. At a higher CC content, maximum energy was required to pull off and thus the maximum adhesive strength was recorded with 2.0 wt% CC content. The adhesive strength increased almost 25% after adding 2.0 wt% CC in WBPU adhesive material. The adhesive strength was also checked at moderately high temperatures. The adhesive strength dropped 72% of WBPU. However, both CC and HQ worked against dropping the adhesive strength. Both WBPU-CC and WBPU-HQ series showed higher adhesive strength than the pristine WBPU adhesive at 80 °C. The maximum resistance was found with higher CC or HQ content (2.0 wt%). From the adhesive strength values, it was also shown that the decrease rate was always higher for HQ than for CC. At 80 °C, the adhesive strength decreased 41% and 17% for HQ and CC, respectively. The hydrogen bond worked against the dropping and it kept strongly the mechanical encoring with nylon fabrics [
10]. The hydrogen bond still worked strongly to oppose the dropping of adhesive strength at this temperature. The hydrogen bond energy was at a maximum in the WBPU-CC-200 sample (2.0 wt% CC), and thus the mechanical interlocking was strong enough even at that temperature; eventually, the adhesive strength was slightly affected at 80 °C for the WBPU-CC-200 sample.