Effects of Fly Ash and Hexadecyltrimethoxysilane on the Compressive Properties and Water Resistance of Magnesium Oxychloride Cement
Abstract
:1. Introduction
2. Materials and Mix Design
2.1. Raw Materials
2.2. Mix Design
3. Experimental Program
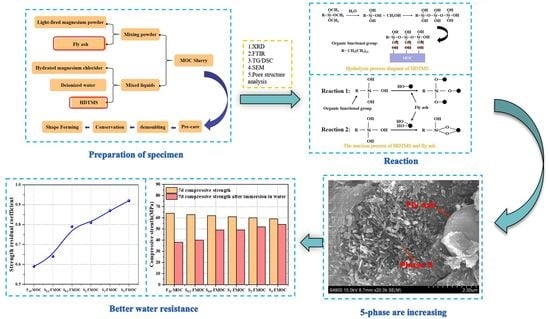
3.1. Preparation of Specimens
3.2. Methods of Testing
3.2.1. Setting Time and Fluidity Tests
3.2.2. Compressive Strength and Water Resistance
3.2.3. Microstructural Characterization
- (1)
- X-ray diffraction (XRD)
- (2)
- Fourier transform infrared spectroscopy (FTIR)
- (3)
- Thermal analyses
- (4)
- Scanning electron microscopy test (SEM)
- (5)
- Pore size distribution analyses
4. Results and Discussion
4.1. Effects of Fly Ash on MOC
4.1.1. Setting Time and Fluidity
4.1.2. Compressive Strength
4.1.3. Water Resistance
4.2. Effects of Fly Ash and HDTMS on MOC
4.2.1. Setting Time and Fluidity
4.2.2. Compressive Strength and Strength Residual Coefficient Test
4.2.3. X-ray Diffraction (XRD)
4.2.4. FTIR Analysis
4.2.5. TG and DSC Analysis
4.2.6. SEM Observation
4.2.7. Pore Structure Analysis
5. Conclusions
- Mixing fly ash into MOC obviously increased its setting time and fluidity. The spatial reticulation structure of MOC is the main reason for its poor water resistance. The strength residual coefficient of the control sample was only 0.45, while 20% fly ash improved the water resistance of MOC by increasing the strength residual coefficient to 0.59.
- The compounding of fly ash and HDTMS in MOC prolonged its setting time while enhancing its flow properties, mainly related to the hydrolysis products of HDTMS and its coupling products with other raw materials. Although the compressive strength of the modified specimens of MOC was reduced after compounding, the strength values still remained around 50–65 MPa. However, the residual coefficient of strength of MOC with fly ash and HDTMS in a water environment could be maintained above 0.8.
- Microscopic analysis allowed us to determine that compounded fly ash and HDTMS promote the formation of hydration product 5-phase and Mg–Cl–Si–H gel, increasing the density of the internal structure of MOC. Furthermore, HDTMS can delay the reaction between water and 5-phase, such that 5-phase can be better retained. At the same time, HDTMS hydrophobically modifies the fly ash, which is beneficial for the uniform dispersion of modified fly ash, blocking the connected pores and reducing moisture intrusion. According to the pore structure analysis, it was found that the harmful pores were significantly reduced, while the proportion of small-sized pores increased. This refined pore structure also contributes to the enhanced water resistance of MOC.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, T.; Yuan, Q.; Deng, D. The role of phosphoric acid in improving the strength of magnesium oxychloride cement pastes with large molar ratios of H2O/MgCl2. Cem. Concr. Compos. 2019, 97, 379–386. [Google Scholar] [CrossRef]
- Ma, H.; Guan, B.; Wang, Y.; Liu, K.; Zhang, J. Research progress on magnesium oxychloride cement cementitious materials. Mater. Rep. 2015, 29, 103–107. (In Chinese) [Google Scholar]
- Sun, X.; Ye, Q.; Zhou, W.; Han, Y.; Gong, S.; Zhou, W.; Shi, S.Q.; Li, J.; Fang, Z. Tannin-modified magnesium oxychloride cement with high-strength and reinforced water-resistance. J. Clean. Prod. 2022, 374, 133543. [Google Scholar] [CrossRef]
- Aiken, T.A.; Kwasny, J.; Russell, M.; McPolin, D.; Bagnall, L. Effect of partial MgO replacement on the properties of magnesium oxychloride cement. Cem. Concr. Compos. 2022, 134, 104791. [Google Scholar] [CrossRef]
- He, P.; Poon, C.S.; Richardson, I.G.; Tsang, D.C. The mechanism of supplementary cementitious materials enhancing the water resistance of magnesium oxychloride cement (MOC): A comparison between pulverized fuel ash and incinerated sewage sludge ash. Cem. Concr. Compos. 2020, 109, 103562. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.; Zhang, X.; Wang, X.; Zhang, A. Raw material ratio optimisation of magnesium oxychloride cement using response surface method. Constr. Build. Mater. 2021, 272, 121648. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, W.; Zhang, W.; Li, J.; Chen, H. Tuning the phase structure and mechanical performance of magnesium oxychloride cements by curing temperature and H2O/MgCl2 ratio. Constr. Build. Mater. 2018, 179, 413–419. [Google Scholar] [CrossRef]
- Feng, C.; Guan, B.; Zhang, B.; Chen, H.; Fang, J. Effect of humidity on carbon dioxide adsorption capacity of magnesium oxychloride cement. Bull. Chin. Ceram. Soc. 2018, 37, 2355–2360. (In Chinese) [Google Scholar]
- He, P.; Poon, C.S.; Tsang, D.C. Effect of pulverized fuel ash and CO2 curing on the water resistance of magnesium oxychloride cement (MOC). Cem. Concr. Res. 2017, 97, 115–122. [Google Scholar] [CrossRef]
- Gong, W.; Wang, N.; Zhang, N. Effect of fly ash and metakaolin on the macroscopic and microscopic characterizations of magnesium oxychloride cement. Constr. Build. Mater. 2021, 267, 120957. [Google Scholar] [CrossRef]
- El-Feky, M.; Mohsen, A.; El-Tair, A.M.; Kohail, M. Microstructural investigation for micro- nano-silica engineered magnesium oxychloride cement. Constr. Build. Mater. 2022, 342, 127976. [Google Scholar] [CrossRef]
- Xu, A.; Chen, H.; Guan, B.; Feng, C. Early hydration process of magnesium oxychloride cement with mineral admixture. Appl. Chem. Ind. 2017, 46, 265–269. (In Chinese) [Google Scholar]
- Guo, Y.; Zhang, Y.; Soe, K. Effect of sodium monofluorophosphate and phosphates on mechanical properties and water resistance of magnesium oxychloride cement. Cem. Concr. Compos. 2022, 129, 104472. [Google Scholar] [CrossRef]
- Yang, T.; Guan, B.; Wang, Y.; Yang, X.; Chen, H.; Zhang, Y. Impact of limestone powder on properties of magnesium oxychloride cement. Mater. Rep. 2015, 29, 128–132. (In Chinese) [Google Scholar]
- Wu, J.; Guan, B.; Chen, H.; Tian, H.; Liu, J.; Xiong, R. Effects of polycarboxylate superplasticiser on the early hydration properties of magnesium oxychloride cement. Constr. Build. Mater. 2020, 259, 119862. [Google Scholar] [CrossRef]
- Ye, Q.; Han, Y.; Zhang, S.; Gao, Q.; Zhang, W.; Chen, H.; Gong, S.; Shi, S.Q.; Xia, C.; Li, J. Bioinspired and biomineralized magnesium oxychloride cement with enhanced compressive strength and water resistance. J. Hazard. Mater. 2020, 383, 121099. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Guan, B.; Zhang, B.; Chen, H.; Fang, J. Moisture erosion behavior of magnesium oxychloride cement with the action of water reducer. Non-Met. Mines 2019, 42, 41–44. (In Chinese) [Google Scholar]
- Reddy, K.J.; John, S.; Weber, H.; Argyle, M.D.; Bhattacharyya, P.; Taylor, D.T.; Christensen, M.; Foulke, T.; Fahlsing, P. Simultaneous capture and mineralization of coal combustion flue gas carbon dioxide (CO2). Energy Procedia 2011, 4, 1574–1583. [Google Scholar] [CrossRef] [Green Version]
- Chau, C.; Chan, J.; Li, Z. Influences of fly ash on magnesium oxychloride mortar. Cem. Concr. Compos. 2009, 31, 250–254. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.; Wang, X.; Zhang, X.; Zhang, J.; Xie, H.; Zhang, A. Superhydrophobic magnesium oxychloride cement based composites with integral stability and recyclability. Cem. Concr. Compos. 2021, 118, 103973. [Google Scholar] [CrossRef]
- Huang, J.; Ge, S.; Wang, H.; Chen, R. Study on the improvement of water resistance and water absorption of magnesium oxychloride cement using long-chain organosilane-nonionic surfactants. Constr. Build. Mater. 2021, 306, 124872. [Google Scholar] [CrossRef]
- WB/T 1019–2002; Caustic Burned Magnesia for Magnesium Oxychloride Cement Products. State Economic and Trade Commission of the People’s Republic of China: Beijing, China, 2002.
- Guo, Y.; Zhang, Y.; Soe, K.; Wuhrer, R.; Hutchison, W.D.; Timmers, H. Development of magnesium oxychloride cement with enhanced water resistance by adding silica fume and hybrid fly ash-silica fume. J. Clean. Prod. 2021, 313, 127682. [Google Scholar] [CrossRef]
- Wu, J.; Chen, H.; Guan, B.; Xia, Y.; Sheng, Y.; Fang, J. Effect of fly ash on rheological properties of magnesium oxychloride cement. J. Mater. Civ. Eng. 2019, 31, 04018405. [Google Scholar] [CrossRef]
- GB/T 8077–2012; Methods for Testing Uniformity of Concrete Admixture. Chinese Standards Association: Beijing, China, 2012.
- GB/T 50080–2011; Water Consumption, Setting Time and Stability Test Method for Standard Consistency of Cement. Chinese Standards Association: Beijing, China, 2011.
- Tang, S.; Hu, Y.; Ren, W.; Yu, P.; Huang, Q.; Qi, X.; Li, Y.; Chen, E. Modeling on the hydration and leaching of eco-friendly magnesium oxychloride cement paste at the micro-scale. Constr. Build. Mater. 2019, 204, 684–690. [Google Scholar] [CrossRef]
- Gurney, L.R.; Bentz, D.P.; Sato, T.; Weiss, W.J. Reducing set retardation in high-volume fly ash mixtures with the use of limestone: Improving constructability for sustainability. Transp. Res. Board 2012, 2290, 139–146. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, L.; Yu, J.; Yu, K. Mechanical properties of high ductile magnesium oxychloride cement-based composites after water soaking. Cem. Concr. Compos. 2019, 97, 248–258. [Google Scholar] [CrossRef]
- Suraneni, P.; Hajibabaee, A.; Ramanathan, S.; Wang, Y.; Weiss, J. New insights from reactivity testing of supplementary cementitious materials. Cem. Concr. Compos. 2019, 103, 331–338. [Google Scholar] [CrossRef]
- Wang, X. Effect of fly ash on properties evolution of cement based materials. Constr. Build. Mater. 2014, 69, 32–40. [Google Scholar] [CrossRef]
- Gong, W.; Wang, N.; Zhang, N.; Han, W.; Qiao, H. Water resistance and a comprehensive evaluation model of magnesium oxychloride cement concrete based on Taguchi and entropy weight method. Constr. Build. Mater. 2020, 260, 119817. [Google Scholar] [CrossRef]
- Zhang, C.; Deng, D. Research on water resistance of magnesium oxychloride cement and its improvement. J. Chin. Ceram. Soc. 1995, 23, 673–679. (In Chinese) [Google Scholar]
- Ran, L.; Fan, B.; Lan, L. Silane coupling agent modified fly ash and its application in polyurethane. Thermosetting Resin 2020, 35, 21–25+29. [Google Scholar]
- Liu, B.; Shi, J.; Sun, M.; He, Z.; Xu, H.; Tan, J. Mechanical and permeability properties of polymer-modified concrete using hydrophobic agent. J. Build. Eng. 2020, 31, 101337. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; Abd El-Aleem, S.; Amer, A.A.; El-Didamony, H.; Arif, M.A. Combined impact of silicate-amorphicity and MgO-reactivity on the performance of Mg-silicate cement. Constr. Build. Mater. 2018, 189, 78–85. [Google Scholar] [CrossRef]


















| Material | MgO | SiO2 | Al2O3 | CaO | Fe2O3 | Na2O | K2O | P2O5 |
|---|---|---|---|---|---|---|---|---|
| MgO | 85.21% | 4.87% | 2.01% | 1.58% | 1.32% | - | - | 0.21% |
| Fly ash | 0.98% | 45.32% | 35.12% | 3.25% | 5.12% | 0.35% | 1.58% | - |
| Component | MgCl2·6H2O | SO42− | PO42− | Ca2+ | Fe2O3 |
|---|---|---|---|---|---|
| Mass fraction | ≥98% | ≤0.005% | ≤0.001% | ≤0.05% | ≤0.0005% |
| Mixture | Molar Ratio in All Mixtures | Fly Ash * | HDTMS | |
|---|---|---|---|---|
| MgO/MgCl2 | H2O/MgCl2 | |||
| MOC (reference sample) | 8 | 13 | 0 | 0 |
| F10-MOC | 8 | 13 | 10 | 0 |
| F20-MOC | 8 | 13 | 20 | 0 |
| F30-MOC | 8 | 13 | 30 | 0 |
| F40-MOC | 8 | 13 | 40 | 0 |
| S0.1-FMOC | 8 | 13 | 20 | 0.1 |
| S0.5-FMOC | 8 | 13 | 20 | 0.5 |
| S1-FMOC | 8 | 13 | 20 | 1 |
| S2-FMOC | 8 | 13 | 20 | 2 |
| S3-FMOC | 8 | 13 | 20 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, B.; He, Z.; Wei, F.; Wang, F.; Yu, J. Effects of Fly Ash and Hexadecyltrimethoxysilane on the Compressive Properties and Water Resistance of Magnesium Oxychloride Cement. Polymers 2023, 15, 172. https://doi.org/10.3390/polym15010172
Guan B, He Z, Wei F, Wang F, Yu J. Effects of Fly Ash and Hexadecyltrimethoxysilane on the Compressive Properties and Water Resistance of Magnesium Oxychloride Cement. Polymers. 2023; 15(1):172. https://doi.org/10.3390/polym15010172
Chicago/Turabian StyleGuan, Bowen, Zhenqing He, Fulu Wei, Faping Wang, and Jincheng Yu. 2023. "Effects of Fly Ash and Hexadecyltrimethoxysilane on the Compressive Properties and Water Resistance of Magnesium Oxychloride Cement" Polymers 15, no. 1: 172. https://doi.org/10.3390/polym15010172
APA StyleGuan, B., He, Z., Wei, F., Wang, F., & Yu, J. (2023). Effects of Fly Ash and Hexadecyltrimethoxysilane on the Compressive Properties and Water Resistance of Magnesium Oxychloride Cement. Polymers, 15(1), 172. https://doi.org/10.3390/polym15010172