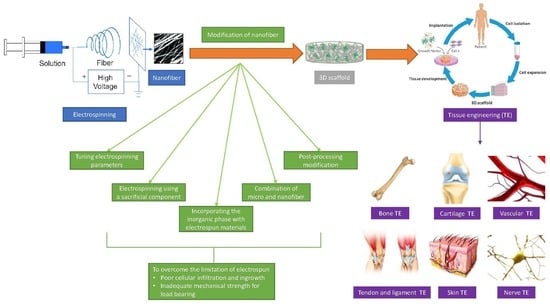
Overview of Electrospinning for Tissue Engineering Applications
Abstract
:1. Introduction
2. Electrospinning Technique
Development of Electrospinning
3. Polymers Used in Electrospinning
3.1. Natural and Synthetic Polymers
3.2. Copolymers
4. Solvent Used for Electrospinning
| Polymer | Solvent | Voltage | Fibre Diameter (nm) | Application | Reference |
|---|---|---|---|---|---|
| Silk fibroin/PCL/polyglycerol sebacate | 1,1,1,3,3,3-Hexafluoro-2-propanol, formic acid | DC | 4100 ± 3000–2110 ± 1340 | Skin TE | [34] |
| PCL/HA/gelatin | Chloroform, methanol | DC | 615 ± 269 | Bone TE | [32] |
| Chitosan | Trifluoroacetic acid, dichloromethane | DC | 231.2 ± 93.3 | Bone regeneration | [35] |
| Collagen/polypyrrole/chitosan | Water/ethanol mixture | DC | 337.9–83.7 | Cardiac TE | [43] |
| Polyethylene glycol/acetate butyrate | Acetone, dimethylacetamide | DC | 436.81 ± 139.52 | Scaffold TE | [44] |
| PCL | Acetic acid/formic acid/acetone | AC | 960 ± 400–2100 ± 1900 | Industrial scale fabrication | [45] |
| Polyurethane/polyamide 6 (PA6) | - | AC | - | Suture | [46] |
| Fish skin gelatin (FG)/PCL | Glacial acetic acid | AC | 237–313 | Biomaterial | [47] |
| PCL | Formic acid, formic acid/acetic acid, and formic acid/acetic acid/acetone | AC | - | Industrial scale fabrication | [48] |
5. Mechanism of Electrospinning
6. Electrospinning Parameters
7. Electrospun Nanofibres for TE Application

7.1. Bone TE
7.2. Cartilage TE
7.3. Vascular TE
7.4. Nerve TE
7.5. Skin TE
7.6. Tendon and Ligament TE
8. Clinical Perspectives of Electrospun Nanofibres
9. Limitation of Electrospun Scaffold
9.1. Poor Cellular Infiltration and Ingrowth
9.2. Inadequate Mechanical Strength for Load Bearing
10. Solution for Overcoming the Electrospun Scaffold Limitation
10.1. Tuning Electrospinning Parameters
10.2. Electrospinning Using a Sacrificial Component
10.3. Combination of Micro- and Nanofibres
10.4. Incorporating the Inorganic Phase with Electrospun Materials
10.5. Postprocessing Modification
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasita, R.; Katti, D.S. Nanofibers and their applications in tissue engineering. Int. J. Nanomed. 2006, 1, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.A.; Ma, P.X. Nano-fibrous scaffolds for tissue engineering. Colloids Surf. B Biointerfaces 2004, 39, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Nastyshyn, S.; Stetsyshyn, Y.; Raczkowska, J.; Nastishin, Y.; Melnyk, Y.; Panchenko, Y.; Budkowski, A. Temperature-Responsive Polymer Brush Coatings for Advanced Biomedical Applications. Polymers 2022, 14, 4245. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.K.; Tuan, R.S. Tissue Engineering with Mesenchymal Stem Cells. IEEE Eng. Med. Biol. Mag. 2003, 22, 51–56. [Google Scholar] [CrossRef]
- Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Sequeira, R.S.; Miguel, S.P.; Cabral, C.S.D.; Moreira, A.F.; Ferreira, P.; Correia, I.J. Development of a poly(vinyl alcohol)/lysine electrospun membrane-based drug delivery system for improved skin regeneration. Int. J. Pharm. 2019, 570, 118640. [Google Scholar] [CrossRef]
- García-Salinas, S.; Evangelopoulos, M.; Gámez-Herrera, E.; Arruebo, M.; Irusta, S.; Taraballi, F.; Mendoza, G.; Tasciotti, E. Electrospun anti-inflammatory patch loaded with essential oils for wound healing. Int. J. Pharm. 2020, 577, 119067. [Google Scholar] [CrossRef]
- Eivazi Zadeh, Z.; Solouk, A.; Shafieian, M.; Haghbin Nazarpak, M. Electrospun polyurethane/carbon nanotube composites with different amounts of carbon nanotubes and almost the same fiber diameter for biomedical applications. Mater. Sci. Eng. 2021, 118, 111403. [Google Scholar] [CrossRef]
- Zhang, W.; He, Z.; Han, Y.; Jiang, Q.; Zhan, C.; Zhang, K.; Li, Z.; Zhang, R. Structural design and environmental applications of electrospun nanofibers. Compos. Part A Appl. Sci. Manuf. 2020, 137, 106009. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Antioxidant, antibacterial and antifungal electrospun nanofibers for food packaging applications. Food Res. Int. 2020, 130, 108927. [Google Scholar] [CrossRef]
- Moazzami Goudarzi, Z.; Behzad, T.; Ghasemi-Mobarakeh, L.; Kharaziha, M. An investigation into influence of acetylated cellulose nanofibers on properties of PCL/Gelatin electrospun nanofibrous scaffold for soft tissue engineering. Polymer 2021, 213, 123313. [Google Scholar] [CrossRef]
- Chandika, P.; Heo, S.Y.; Kim, T.H.; Oh, G.W.; Kim, G.H.; Kim, M.S.; Jung, W.K. Recent advances in biological macromolecule based tissue-engineered composite scaffolds for cardiac tissue regeneration applications. Int. J. Biol. Macromol. 2020, 164, 2329–2357. [Google Scholar] [CrossRef]
- Cao, S.; Zhao, Y.; Hu, Y.; Zou, L.; Chen, J. New perspectives: In-situ tissue engineering for bone repair scaffold. Compos. Part B Eng. 2020, 202, 108445. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Chen, Y.; Wang, X.; Xu, X.; Shen, Y.; Khan, A.u.R.; Aldalbahi, A.; Fetz, A.E.; Bowlin, G.L.; El-Newehy, M.; et al. Electrospinning nanofiber scaffolds for soft and hard tissue regeneration. J. Mater. Sci. Technol. 2020, 59, 243–261. [Google Scholar] [CrossRef]
- Chen, Y.; Shafiq, M.; Liu, M.; Morsi, Y.; Mo, X. Advanced fabrication for electrospun three-dimensional nanofiber aerogels and scaffolds. Bioact. Mater. 2020, 5, 963–979. [Google Scholar] [CrossRef]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Kumar, A.; Jacob, A. Techniques in scaffold fabrication process for tissue engineering applications: A review. J. Appl. Biol. Biotechnol. 2022, 10, 163–176. [Google Scholar] [CrossRef]
- Peck, M.; Dusserre, N.; McAllister, T.N.; L’Heureux, N. Tissue engineering by self-assembly. Mater. Today 2011, 14, 218–224. [Google Scholar] [CrossRef]
- Tucker, N.; Stanger, J. The History of the Science and Technology of Electrospinning from 1600 to 1995. J. Eng. Fibers Fabr. 2012, 7, 63–73. [Google Scholar] [CrossRef]
- Zulkifli, M.Z.A.; Kamarudin, N.H.N.; Nordin, D. Polycaprolactone / Chlorophyllin Sodium Copper Salt Nanofibrous Mats Prepared by Electrospinning for Soft Tissue Engineering. J. Kejuruter. 2019, 2, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Kang, S.X.; Yang, Y.Y.; Yu, D.G. Electrospun functional nanofiber membrane for antibiotic removal in water: Review. Polymers 2021, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Taylor, G. Electrically driven jets. Proc. R. Soc. London. A Math. Phys. Sci. 1969, 313, 453–475. [Google Scholar]
- Goh, Y.F.; Shakir, I.; Hussain, R. Electrospun fibers for tissue engineering, drug delivery, and wound dressing. J. Mater. Sci. 2013, 48, 3027–3054. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun nanofibers: Solving global issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Tao, D.; Higaki, Y.; Ma, W.; Wu, H.; Shinohara, T.; Yano, T.; Takahara, A. Chain orientation in poly(glycolic acid)/halloysite nanotube hybrid electrospun fibers. Polymer 2015, 60, 284–291. [Google Scholar] [CrossRef]
- Xu, J.; Fang, Q.; Liu, Y.; Zhou, Y.; Ye, Z.; Tan, W.S. In situ ornamenting poly(ε-caprolactone) electrospun fibers with different fiber diameters using chondrocyte-derived extracellular matrix for chondrogenesis of mesenchymal stem cells. Colloids Surf. B Biointerfaces 2021, 197, 111374. [Google Scholar] [CrossRef]
- Shakhssalim, N.; Soleimani, M.; Dehghan, M.M.; Rasouli, J.; Taghizadeh-Jahed, M.; Torbati, P.M.; Naji, M. Bladder smooth muscle cells on electrospun poly(ε-caprolactone)/poly(L-lactic acid) scaffold promote bladder regeneration in a canine model. Mater. Sci. Eng. 2017, 75, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Sharma, C.; Purohit, S.D.; Singh, H.; Dinda, A.K.; Potdar, P.D.; Chou, C.F.; Mishra, N.C. Gelatin-polycaprolactone-nanohydroxyapatite electrospun nanocomposite scaffold for bone tissue engineering. Mater. Sci. Eng. 2021, 119, 111588. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.B.; Fajardo, A.R.; Gerola, A.P.; Rodrigues, J.H.S.; Nakamura, C.V.; Muniz, E.C.; Hsieh, Y.L. First report of electrospun cellulose acetate nanofibers mats with chitin and chitosan nanowhiskers: Fabrication, characterization, and antibacterial activity. Carbohydr. Polym. 2020, 250, 116954. [Google Scholar] [CrossRef]
- Keirouz, A.; Zakharova, M.; Kwon, J.; Robert, C.; Koutsos, V.; Callanan, A.; Chen, X.; Fortunato, G.; Radacsi, N. High-throughput production of silk fibroin-based electrospun fibers as biomaterial for skin tissue engineering applications. Mater. Sci. Eng. 2020, 112, 110939. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Fujiwara, T.; Anderson, K.M.; Karydis, A.; Ghadri, M.N.; Bumgardner, J.D. A comparison of two types of electrospun chitosan membranes and a collagen membrane in vivo. Dent. Mater. 2021, 37, 60–70. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-ε-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef]
- Wang, M.; Hsieh, A.J.; Rutledge, G.C. Electrospinning of poly(MMA-co-MAA) copolymers and their layered silicate nanocomposites for improved thermal properties. Polymer 2005, 46, 3407–3418. [Google Scholar] [CrossRef]
- Hasan, M.M.; Alam, A.K.M.M.; Nayem, K.A. Application of Electrospinning Techniques for the Production of Tissue Engineering Scaffolds: A Review. Eur. Sci. J. 2014, 10, 1857–7881. [Google Scholar]
- Venugopal, J.; Low, S.; Choon, A.T.; Ramakrishna, S. Interaction of Cells and Nanofiber Scaffolds in Tissue Engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 84, 34–48. [Google Scholar] [CrossRef]
- Fomby, P.; Cherlin, A.J.; Hadjizadeh, A.; Doillon, C.J.; Sueblinvong, V.; Weiss, D.J.; Bates, J.H.T.; Gilbert, T.; Liles, W.C.; Lutzko, C.; et al. A review of key challenges of electrospun scaffolds for tissue-engineering applications. Ann. Am. Thorac. Soc. 2010, 12, 181–204. [Google Scholar]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Erencia, M.; Cano, F.; Tornero, J.A.; Fernandes, M.M.; Tzanov, T.; Macanás, J.; Carrillo, F. Electrospinning of gelatin fibers using solutions with low acetic acid concentration: Effect of solvent composition on both diameter of electrospun fibers and cytotoxicity. J. Appl. Polym. Sci. 2015, 132, 42115. [Google Scholar] [CrossRef]
- Zarei, M.; Samimi, A.; Khorram, M.; Abdi, M.M.; Golestaneh, S.I. Fabrication and characterization of conductive polypyrrole/chitosan/collagen electrospun nanofiber scaffold for tissuee engineering application. Int. J. Biol. Macromol. 2021, 168, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Kai, D.; Pasbakhsh, P.; Teow, S.Y.; Lim, Y.Y.; Pushpamalar, J. Electrospun cellulose acetate butyrate/polyethylene glycol (CAB/PEG) composite nanofibers: A potential scaffold for tissue engineering. Colloids Surf. B Biointerfaces 2020, 188, 110713. [Google Scholar] [CrossRef]
- Sivan, M.; Madheswaran, D.; Valtera, J.; Kostakova, E.K.; Lukas, D. Alternating current electrospinning: The impacts of various high-voltage signal shapes and frequencies on the spinnability and productivity of polycaprolactone nanofibers. Mater. Des. 2022, 213, 110308. [Google Scholar] [CrossRef]
- Madheswaran, D.; Sivan, M.; Valtera, J.; Kostakova, K.E.; Egghe, T.; Asadian, M.; Novotny, V.; Nguyen, A.H.N.; Sevcu, A.; Morent, R.; et al. Composite yarns with antibacterial nanofibrous sheaths produced by collectorless alternating-current electrospinning for suture applications. J. Appl. Polym. Sci. 2021, 139, 51851. [Google Scholar] [CrossRef]
- Lacy, H.A.; Jenčová, V.; Lukáš, D.; Stanishevsky, A. Alternating field electrospinning of blended fish gelatin/poly(ε-caprolactone) nanofibers. Mater. Lett. 2023, 341, 134284. [Google Scholar] [CrossRef]
- Sivan, M.; Madheswaran, D.; Hauzerova, S.; Novotny, V.; Hedvicakova, V.; Jencova, V.; Kostakova, K.E.; Schindler, M.; Lukas, D. AC electrospinning: Impact of high voltage and solvent on the electrospinnability and productivity of polycaprolactone electrospun nanofibrous scaffolds. Mater. Chem. 2022, 26, 101025. [Google Scholar] [CrossRef]
- Chew, S.; Wen, Y.; Dzenis, Y.; Leong, K. The Role of Electrospinning in the Emerging Field of Nanomedicine. Curr. Pharm. Des. 2006, 12, 4751–4770. [Google Scholar] [CrossRef]
- Isaac, B.; Taylor, R.M.; Reifsnider, K. Mechanical and Dielectric Properties of Aligned Electrospun Fibers. Fibers 2021, 9, 4. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, J.; Chen, S.; Wu, S. Designing an Innovative Electrospinning Strategy to Generate PHBV Nanofiber Scaffolds with a Radially Oriented Fibrous Pattern. Nanomaterials 2023, 13, 1150. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Fang, D.; Hsiao, B.S.; Chu, B.; Chen, W. Optimization and Characterization of Dextran Membranes Prepared by Electrospinning. Biomacromolecules 2004, 5, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Nagapudi, K.; Apkarian, R.P.; Chaikof, E.L. Engineered collagen—PEO nanofibers and fabrics. J. Biomater. Sci. Polym. Ed. 2001, 12, 979–993. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, H.; Lee, S.-H.; Sigmund, W.M. Poly (acrylic acid) nanofibers by electrospinning. Mater. Lett. 2005, 59, 829–832. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. The effects of solution properties and polyelectrolyte on electrospinning of ultrafine poly(ethylene oxide) fibers. Polymer 2004, 45, 2959–2966. [Google Scholar] [CrossRef]
- Zeng, J.; Xu, X.; Chen, X.; Liang, Q.; Bian, X.; Yang, L.; Jing, X. Biodegradable electrospun fibers for drug delivery. J. Control. Release 2003, 92, 227–231. [Google Scholar] [CrossRef]
- Koski, A.; Yim, K.; Shivkumar, S. Effect of molecular weight on fibrous PVA produced by electrospinning. Mater. Lett. 2004, 58, 493–497. [Google Scholar] [CrossRef]
- Hohman, M.M.; Shin, M.; Rutledge, G.; Brenner, M.P. Electrospinning and electrically forced jets. II. Applications. Phys. Fluids 2001, 13, 2221–2236. [Google Scholar] [CrossRef]
- Demir, M.M.; Yilgor, I.; Yilgor, E.; Erman, B. Electrospinning of polyurethane fibers. Polymer 2002, 43, 3303–3309. [Google Scholar] [CrossRef]
- Lee, C.H.; Shin, H.J.; Cho, I.H.; Kang, Y.M.; Kim, I.A.; Park, K.D.; Shin, J.W. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials 2005, 26, 1261–1270. [Google Scholar] [CrossRef]
- Geng, X.; Kwon, O.H.; Jang, J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials 2005, 26, 5427–5432. [Google Scholar] [CrossRef]
- Mit-uppatham, C.; Nithitanakul, M.; Supaphol, P. Ultratine electrospun polyamide-6 fibers: Effect of solution conditions on morphology and average fiber diameter RID C-4353-2008. Macromol. Chem. Phys. 2004, 205, 2327–2338. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Prasath Mani, M.; Ayyar, M.; Rathanasamy, R. Biomimetic electrospun polyurethane matrix composites with tailor made properties for bone tissue engineering scaffolds. Polym. Test. 2019, 78, 105955. [Google Scholar] [CrossRef]
- Nemati, S.; Kim, S.-J.; Shin, Y.M.; Shin, H. Current progress in application of polymeric nanofibers to tissue engineering. Nano Converg. 2019, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Rethinam, S.; Basaran, B.; Vijayan, S.; Mert, A.; Bayraktar, O.; Aruni, A.W. Electrospun nano-bio membrane for bone tissue engineering application- a new approach. Mater. Chem. Phys. 2020, 249, 123010. [Google Scholar] [CrossRef]
- Sharifi, F.; Irani, S.; Azadegan, G.; Pezeshki-Modaress, M.; Zandi, M.; Saeed, M. Co-electrospun gelatin-chondroitin sulfate/polycaprolactone nanofibrous scaffolds for cartilage tissue engineering. Bioact. Carbohydr. Diet. Fibre 2020, 22, 100215. [Google Scholar] [CrossRef]
- Feng, B.; Ji, T.; Wang, X.; Fu, W.; Ye, L.; Zhang, H.; Li, F. Engineering cartilage tissue based on cartilage-derived extracellular matrix cECM/PCL hybrid nanofibrous scaffold. Mater. Des. 2020, 193, 108773. [Google Scholar] [CrossRef]
- Silva, J.C.; Udangawa, R.N.; Chen, J.; Mancinelli, C.D.; Garrudo, F.F.F.; Mikael, P.E.; Cabral, J.M.S.; Ferreira, F.C.; Linhardt, R.J. Kartogenin-loaded coaxial PGS/PCL aligned nanofibers for cartilage tissue engineering. Mater. Sci. Eng. 2020, 107, 110291. [Google Scholar] [CrossRef]
- Joy, J.; Pereira, J.; Aid-Launais, R.; Pavon-Djavid, G.; Ray, A.R.; Letourneur, D.; Meddahi-Pellé, A.; Gupta, B. Gelatin—Oxidized carboxymethyl cellulose blend based tubular electrospun scaffold for vascular tissue engineering. Int. J. Biol. Macromol. 2018, 107, 1922–1935. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Bartnikowski, M.; Hamlet, S.; Ivanovski, S. Electrospun biphasic tubular scaffold with enhanced mechanical properties for vascular tissue engineering. Mater. Sci. Eng. 2018, 82, 10–18. [Google Scholar] [CrossRef]
- Xiang, P.; Wang, S.S.; He, M.; Han, Y.H.; Zhou, Z.H.; Chen, D.L.; Li, M.; Ma, L.Q. The in vitro and in vivo biocompatibility evaluation of electrospun recombinant spider silk protein/PCL/gelatin for small caliber vascular tissue engineering scaffolds. Colloids Surf. B Biointerfaces 2018, 163, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Vogt, L.; Rivera, L.R.; Liverani, L.; Piegat, A.; El Fray, M.; Boccaccini, A.R. Poly(ε-caprolactone)/poly(glycerol sebacate) electrospun scaffolds for cardiac tissue engineering using benign solvents. Mater. Sci. Eng. 2019, 103, 109712. [Google Scholar] [CrossRef] [PubMed]
- Dippold, D.; Tallawi, M.; Tansaz, S.; Roether, J.A.; Boccaccini, A.R. Novel electrospun poly(glycerol sebacate)-zein fiber mats as candidate materials for cardiac tissue engineering. Eur. Polym. J. 2016, 75, 504–513. [Google Scholar] [CrossRef]
- Tamimi, M.; Rajabi, S.; Pezeshki-Modaress, M. Cardiac ECM/chitosan/alginate ternary scaffolds for cardiac tissue engineering application. Int. J. Biol. Macromol. 2020, 164, 389–402. [Google Scholar] [CrossRef]
- Heidari, M.; Bahrami, S.H.; Ranjbar-Mohammadi, M.; Milan, P.B. Smart electrospun nanofibers containing PCL/gelatin/graphene oxide for application in nerve tissue engineering. Mater. Sci. Eng. C 2019, 103, 109768. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrospun poly(ε-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Babaie, A.; Bakhshandeh, B.; Abedi, A.; Mohammadnejad, J.; Shabani, I.; Ardeshirylajimi, A.; Reza Moosavi, S.; Amini, J.; Tayebi, L. Synergistic effects of conductive PVA/PEDOT electrospun scaffolds and electrical stimulation for more effective neural tissue engineering. Eur. Polym. J. 2020, 140, 110051. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Park, G.T.; Han, S.S. Electrospun poly(vinyl alcohol)/reduced graphene oxide nanofibrous scaffolds for skin tissue engineering. Colloids Surf. B Biointerfaces 2020, 191, 110994. [Google Scholar] [CrossRef]
- Adeli-Sardou, M.; Yaghoobi, M.M.; Torkzadeh-Mahani, M.; Dodel, M. Controlled release of lawsone from polycaprolactone/gelatin electrospun nano fibers for skin tissue regeneration. Int. J. Biol. Macromol. 2019, 124, 478–491. [Google Scholar] [CrossRef]
- Keirouz, A.; Fortunato, G.; Zhang, M.; Callanan, A.; Radacsi, N. Nozzle-free electrospinning of Polyvinylpyrrolidone/Poly(glycerol sebacate) fibrous scaffolds for skin tissue engineering applications. Med. Eng. Phys. 2019, 71, 56–67. [Google Scholar] [CrossRef]
- Wu, T.; Ding, M.; Shi, C.; Qiao, Y.; Wang, P.; Qiao, R.; Wang, X.; Zhong, J. Resorbable polymer electrospun nanofibers: History, shapes and application for tissue engineering. Chin. Chem. Lett. 2020, 31, 617–625. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Mainardi, V.L.; Talò, G.; McCarthy, A.; John, J.V.; Teusink, M.J.; Hong, L.; Xie, J. Biomaterials with structural hierarchy and controlled 3D nanotopography guide endogenous bone regeneration. Sci. Adv. 2021, 7, eabg3089. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.; Sharma, N.S.; Holubeck, P.A.; Brown, D.; Shah, R.; McGoldrick, D.; John, J.V.; Shahriar, S.M.S.; Xie, J. The Extracellular Matrix Secretion Mechanically Reinforces Interlocking Interfaces. Adv. Mater. 2023, 35, 2207335. [Google Scholar] [CrossRef] [PubMed]
- Nagase, K.; Yamato, M.; Kanazawa, H.; Okano, T. Poly(N-isopropylacrylamide)-based thermoresponsive surfaces provide new types of biomedical applications. Biomaterials 2018, 153, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Klabukov, I.; Balyasin, M.; Krasilnikova, O.; Tenchurin, T.; Titov, A.; Krasheninnikov, M.; Mudryak, D.; Sulina, Y.; Shepelev, A.; Chvalun, S.; et al. Angiogenic Modification of Microfibrous Polycaprolactone by pCMV-VEGF165 Plasmid Promotes Local Vascular Growth after Implantation in Rats. Int. J. Mol. Sci. 2023, 24, 1399. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, K.; Thomas, S.; Kalarikkal, N.; Gnanamani, A. Collagen coated electrospun polycaprolactone (PCL) with titanium dioxide (TiO2) from an environmentally benign solvent: Preliminary physico-chemical studies for skin substitute. J. Polym. Res. 2014, 21, 2–6. [Google Scholar] [CrossRef]
- Ghosal, K.; Manakhov, A.; Zajíčková, L.; Thomas, S. Structural and Surface Compatibility Study of Modified Electrospun Poly(ε-caprolactone) (PCL) Composites for Skin Tissue Engineering. AAPS PharmSciTech 2017, 18, 72–81. [Google Scholar] [CrossRef]
- Cai, J.; Liu, J.; Xu, J.; Li, Y.; Zheng, T.; Zhang, T.; Han, K.; Chen, S.; Jiang, J.; Wu, S. Constructing high-strength nano-micro fibrous woven scaffolds with native-like anisotropic structure and immunoregulatory function for tendon repair and regeneration. Biofabrication 2023, 15, 025002. [Google Scholar] [CrossRef]
- Shahriar, S.M.S.; Mondal, J.; Hasan, M.N.; Revuri, V.; Lee, D.Y.; Lee, Y.K. Electrospinning nanofibers for therapeutics delivery. Nanomaterials 2019, 9, 532. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef]
- Tsiapalis, D.; De Pieri, A.; Biggs, M.; Pandit, A.; Zeugolis, D.I. Biomimetic Bioactive Biomaterials: The Next Generation of Implantable Devices. ACS Biomater. Sci. Eng. 2017, 3, 1172–1174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cao, K.; Zaeri, A.; Zgeib, R.; Chang, C.R. Design, fabrication, and analysis of spatially heterogeneous scaffold by melt electrospinning writing of poly(ε-Caprolactone). J. Appl. Polym. Sci. 2022, 139, 52235. [Google Scholar] [CrossRef]
- Klabukov, I.; Tenchurin, T.; Shepelev, A.; Baranovskii, D.; Mamagulashvili, V.; Dyuzheva, T.; Krasilnikova, O.; Balyasin, M.; Lyundup, A.; Krasheninnikov, M.; et al. Biomechanical Behaviors and Degradation Properties of Multilayered Polymer Scaffolds: The Phase Space Method for Bile Duct Design and Bioengineering. Biomedicines 2023, 11, 745. [Google Scholar] [CrossRef] [PubMed]
- Zander, N.E.; Orlicki, J.A.; Rawlett, A.M.; Beebe, T.P. Electrospun polycaprolactone scaffolds with tailored porosity using two approaches for enhanced cellular infiltration. J. Mater. Sci. Mater. Med. 2013, 24, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Shabani, I.; Haddadi-Asl, V.; Seyedjafari, E.; Soleimani, M. Cellular infiltration on nanofibrous scaffolds using a modified electrospinning technique. Biochem. Biophys. Res. Commun. 2012, 423, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Balguid, A.; Mol, A.; Van Marion, M.H.; Bank, R.A.; Bouten, C.V.C.; Baaijens, F.P.T. Tailoring fiber diameter in electrospun poly(ε-Caprolactone) scaffolds for optimal cellular infiltration in cardiovascular tissue engineering. Tissue Eng. Part A 2009, 15, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Rnjak-Kovacina, J.; Weiss, A.S. Increasing the pore size of electrospun scaffolds. Tissue Eng. Part B Rev. 2011, 17, 365–372. [Google Scholar] [CrossRef]
- Wu, J.; Hong, Y. Enhancing cell infiltration of electrospun fibrous scaffolds in tissue regeneration. Bioact. Mater. 2016, 1, 56–64. [Google Scholar] [CrossRef]
- Kim, T.G.; Chung, H.J.; Park, T.G. Macroporous and nanofibrous hyaluronic acid/collagen hybrid scaffold fabricated by concurrent electrospinning and deposition/leaching of salt particles. Acta Biomater. 2008, 4, 1611–1619. [Google Scholar] [CrossRef]
- Nam, J.; Huang, Y.; Agarwal, S.; Lannutti, J. Improved cellular infiltration in electrospun fiber via engineered porosity. Tissue Eng. 2007, 13, 2249–2257. [Google Scholar] [CrossRef]
- Ju, Y.M.; Choi, J.S.; Atala, A.; Yoo, J.J.; Lee, S.J. Bilayered scaffold for engineering cellularized blood vessels. Biomaterials 2010, 31, 4313–4321. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.; Avegnon, K.L.M.; Holubeck, P.A.; Brown, D.; Karan, A.; Sharma, N.S.; John, J.V.; Weihs, S.; Ley, J.; Xie, J. Electrostatic flocking of salt-treated microfibers and nanofiber yarns for regenerative engineering. Mater. Today Bio 2021, 12, 100166. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun poly (ε-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: Characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 2006, 7, 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.K.; Kadam, S.S.; Soni, V.P.; Bellare, J.R. Improved functionalization of electrospun PLLA/gelatin scaffold by alternate soaking method for bone tissue engineering. Appl. Surf. Sci. 2013, 268, 477–488. [Google Scholar] [CrossRef]
- Elyaderani, A.K.; Lama-Odría, D.; del Carmen, M.; Valle, L.J.d.; Puiggalí, J. Multifunctional Scaffolds Based on Emulsion and Coaxial Electrospinning Incorporation of Hydroxyapatite for Bone Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 15016. [Google Scholar] [CrossRef]
- Baskar, D.; Balu, R.; Kumar, S.S. Mineralization of pristine chitosan film through biomimetic process. Int. J. Biol. Macromol. 2011, 49, 385–389. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Gimeno-Alcañiz, J.V.; Ocio, M.J.; Lagaron, J.M. Comparative performance of electrospun collagen nanofibers cross-linked by means of different methods. ACS Appl. Mater. Interfaces 2009, 1, 218–223. [Google Scholar] [CrossRef]
- Jiang, Q.; Reddy, N.; Yang, Y. Cytocompatible cross-linking of electrospun zein fibers for the development of water-stable tissue engineering scaffolds. Acta Biomater. 2010, 6, 4042–4051. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Q.; Wu, Q. UV-initiated crosslinking of electrospun poly(ethylene oxide) nanofibers with pentaerythritol triacrylate: Effect of irradiation time and incorporated cellulose nanocrystals. Carbohydr. Polym. 2012, 87, 1779–1786. [Google Scholar] [CrossRef]
- Drexler, J.W.; Powell, H.M. Dehydrothermal crosslinking of electrospun collagen. Tissue Eng. Part C Methods 2010, 17, 9–17. [Google Scholar] [CrossRef]
- Owida, H.A.; Al-Nabulsi, J.I.; Alnaimat, F.; Al-Ayyad, M.; Turab, N.M.; Al Sharah, A.; Shakur, M. Recent Applications of Electrospun Nanofibrous Scaffold in Tissue Engineering. Appl. Bionics Biomech. 2022, 2022, 1953861. [Google Scholar] [CrossRef] [PubMed]
- Tebyetekerwa, M.; Ramakrishna, S. What Is Next for Electrospinning? Matter 2020, 2, 279–283. [Google Scholar] [CrossRef]



| Scaffold Fabrication Method | Benefits | Drawbacks | References |
|---|---|---|---|
| Electrospinning |
|
| [18,19] |
| Self-assembly |
|
| [18,20] |
| Phase separation |
|
| [18,19] |
| Parameter | Effects on Morphology of Nanofibre | References |
|---|---|---|
| (1) Solution parameters | ||
| Viscosity | Less number of beads produced; increase in fibre diameter as viscosity increases. | [52,53] |
| Polymer concentration | The fibre diameter increases as the polymer concentration increases. | [54,55,56] |
| Molecular weight of polymer | The number of beads and droplets decreases with an increase in the molecular weight of the polymer. | [57] |
| Conductivity of polymer | Higher conductivity of the polymer causes the fibre diameter to decrease. | [52,57] |
| Surface tension | There is no change in fibre morphology; high surface tension leads to instability of the jet. | [58] |
| (2) Process parameters | ||
| Applied voltage | The fibre diameter decreases as the applied voltage increases. | [56,59] |
| Distance from tip to collector | Large distance from the tip to the collector leads to small production of beads; minimum distance is required to synthesise a uniform fibre. | [60,61] |
| Flow rate | The reduction in fibre diameter is proportional to the reduction in flow rate. | [24] |
| (3) Ambient parameters | ||
| Humidity | High humidity forms pores on the surface of the fibres. | [62] |
| Temperature | An increase in temperature results in a smaller fibre diameter. | [62] |
| Application | Polymer/Material | Solvent | Result | Reference |
|---|---|---|---|---|
| Bone TE |
|
|
| [32] |
|
|
| [63] | |
|
|
| [65] | |
| Cartilage TE |
|
|
| [66] |
|
|
| [67] | |
|
|
| [68] | |
| Vascular TE |
|
|
| [69] |
|
|
| [70] | |
|
|
| [71] | |
| Cardiac TE |
|
|
| [72] |
|
|
| [73] | |
|
|
| [74] | |
| Nerve TE |
|
|
| [75] |
|
|
| [76] | |
|
|
| [77] | |
| Skin TE |
|
|
| [78] |
|
|
| [79] | |
|
|
| [80] |
| Method Used | Polymer | Solvent | Result | Reference |
|---|---|---|---|---|
| Increasing polymer concentration | Polycaprolactone (PCL) | Dimethylformamide (DMF) and Dichloromethane (DCM) |
| [94] |
| Increasing solvent evaporation via heat localisation in the path of fluid jet | Poly(l-lactic acid) (PLLA) | Dichloromethane (DCM) and Dimethylformamide (DMF) |
| [95] |
| Increasing flow rate | Polycaprolactone (PCL) | Chloroform |
| [96] |
| Elastin | Hexafluoroisopropanol |
| [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulkifli, M.Z.A.; Nordin, D.; Shaari, N.; Kamarudin, S.K. Overview of Electrospinning for Tissue Engineering Applications. Polymers 2023, 15, 2418. https://doi.org/10.3390/polym15112418
Zulkifli MZA, Nordin D, Shaari N, Kamarudin SK. Overview of Electrospinning for Tissue Engineering Applications. Polymers. 2023; 15(11):2418. https://doi.org/10.3390/polym15112418
Chicago/Turabian StyleZulkifli, Muhammad Zikri Aiman, Darman Nordin, Norazuwana Shaari, and Siti Kartom Kamarudin. 2023. "Overview of Electrospinning for Tissue Engineering Applications" Polymers 15, no. 11: 2418. https://doi.org/10.3390/polym15112418
APA StyleZulkifli, M. Z. A., Nordin, D., Shaari, N., & Kamarudin, S. K. (2023). Overview of Electrospinning for Tissue Engineering Applications. Polymers, 15(11), 2418. https://doi.org/10.3390/polym15112418