Nanofluids of Amphiphilic Kaolinite-Based Janus Nanosheets for Enhanced Oil Recovery: The Importance of Stable Emulsion
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Intercalation and Exfoliation of Kaolinite
2.2.2. Silanization of Kaolinite Nanosheets
2.2.3. Grafting Polymerization of NIPAAm onto KaolKH Janus Nanosheets
2.3. Characterizations
2.3.1. X-ray Diffraction (XRD)
2.3.2. Fourier Transform Infrared (FT-IR)
2.3.3. Zeta Potential and Dynamic Light Scattering (DLS)
2.3.4. Atomic Force Microscopy (AFM)
2.3.5. Scanning Electron Microscopy (SEM)
2.3.6. Water Contact Angle
2.3.7. Optical Microcopy
2.3.8. Rheology Measurements
2.4. Interfacial Study of KaolKH Janus Nanosheets at Oil–Water Interface
2.4.1. Observation of Interfacial Phenomenon in Oil–Nanosheet–Water System
2.4.2. Elasticity Testing of the Interfacial Film
- Apparent testing and eye observation
- 2.
- Interfacial rheology measurements
2.4.3. Contact Angle of the Interfacial Film
2.5. Core Flooding Test
3. Results and Discussion
3.1. Preparation and Characterizations of Kaolinite-Based Amphiphilic Janus Nanosheets
3.2. Interfacial Behavior of Nanosheets in Oil–Water System
3.2.1. Interfacial Behavior Observations
3.2.2. Rheology of the Interfacial Film
3.2.3. Possible Mechanism for the Formation of Interfacial Film and Climbing Film on Tube Surface
3.2.4. Reversibility between Stable Emulsion and Interfacial Film
3.3. Enhanced Oil Recovery Efficiency of KaolKH Nanofluids
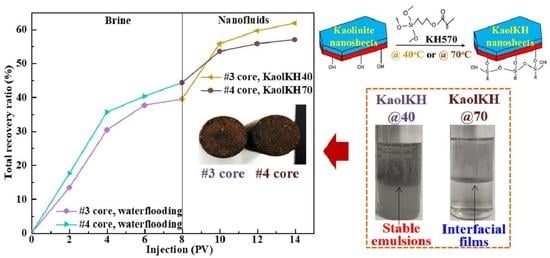
3.3.1. Core Flooding Results
3.3.2. Potential EOR Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Y.X.; Yang, D.H.; Shi, L.C.; Wu, H.Y.; Cao, Y.; He, Y.M.; Xie, T.T. Properties of Nanofluids and Their Applications in Enhanced Oil Recovery: A Comprehensive Review. Energy Fuels 2020, 34, 1202–1218. [Google Scholar] [CrossRef]
- Santos, R.; Loh, W.; Bannwart, A.; Trevisan, O. An overview of heavy oil properties and its recovery and transportation methods. Braz. J. Chem. Eng. 2014, 31, 571–590. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Z.; Li, S.; Jiang, L.; Wang, J.; Wang, P. Utilization of surfactant-stabilized foam for enhanced oil recovery by adding nanoparticles. Energy Fuels 2014, 28, 2384–2394. [Google Scholar] [CrossRef]
- Luo, D.; Wang, F.; Zhu, J.; Tang, L.; Zhu, Z.; Bao, J.; Willson, R.C.; Yang, Z.; Ren, Z. Secondary oil recovery using graphene-based amphiphilic Janus nanosheet fluid at an ultralow concentration. Ind. Eng. Chem. Res. 2017, 56, 11125–11132. [Google Scholar] [CrossRef]
- Ramsden, W. Separation of solids in the surface-layers of solutions and ‘suspensions’ (observations on surface-membranes, bubbles, emulsions, and mechanical coagulation)—Preliminary account. Proc. R. Soc. Lond. 1904, 72, 156–164. [Google Scholar]
- Melle, S.; Lask, M.; Fuller, G.G. Pickering emulsions with controllable stability. Langmuir 2005, 21, 2158–2162. [Google Scholar] [CrossRef] [PubMed]
- Hendraningrat, L.; Li, S.; Torsæter, O. A coreflood investigation of nanofluid enhanced oil recovery. J. Petrol. Sci. Eng. 2013, 111, 128–138. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Li, S.; Torsaeter, O. Enhancing oil recovery of low-permeability Berea sandstone through optimized nanofluids concentration. In Proceedings of the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 2 July 2013. [Google Scholar]
- Hendraningrat, L.; Torsæter, O. Metal oxide-based nanoparticles: Revealing their potential to enhance oil recovery in different wettability systems. Appl. Nanosci. 2015, 5, 181–199. [Google Scholar] [CrossRef]
- Zargartalebi, M.; Kharrat, R.; Barati, N. Enhancement of surfactant flooding performance by the use of silica nanoparticles. Fuel 2015, 143, 21–27. [Google Scholar] [CrossRef]
- Cheraghian, G.; Hendraningrat, L. A review on applications of nanotechnology in the enhanced oil recovery part A: Effects of nanoparticles on interfacial tension. Int. Nano Lett. 2016, 6, 129–138. [Google Scholar] [CrossRef]
- Luo, D.; Wang, F.; Zhu, J.; Cao, F.; Liu, Y.; Li, X.; Willson, R.C.; Yang, Z.; Chu, C.-W.; Ren, Z. Nanofluid of graphene-based amphiphilic Janus nanosheets for tertiary or enhanced oil recovery: High performance at low concentration. Proc. Natl. Acad. Sci. USA 2016, 113, 7711–7716. [Google Scholar] [CrossRef] [PubMed]
- Salem Ragab, A.M.; Hannora, A.E. A Comparative investigation of nano particle effects for improved oil recovery–experimental work. In Proceedings of the SPE Kuwait Oil and Gas Show and Conference, Mishref, Kuwait, 15 October 2015. [Google Scholar]
- Yin, T.; Yang, Z.; Dong, Z.; Lin, M.; Zhang, J. Physicochemical properties and potential applications of silica-based amphiphilic Janus nanosheets for enhanced oil recovery. Fuel 2019, 237, 344–351. [Google Scholar] [CrossRef]
- French, D.J.; Brown, A.T.; Schofield, A.B.; Fowler, J.; Taylor, P.; Clegg, P.S. The secret life of Pickering emulsions: Particle exchange revealed using two colours of particle. Sci. Rep. 2016, 6, 31401. [Google Scholar] [CrossRef]
- Binks, B.P. Particles as surfactants—Similarities and differences. Curr. Opin. Colloid 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Leal-Calderon, F.; Schmitt, V. Solid-stabilized emulsions. Curr. Opin. Colloid 2008, 13, 217–227. [Google Scholar] [CrossRef]
- Sharma, T.; Kumar, G.S.; Sangwai, J.S. Viscoelastic properties of oil-in-water (o/w) Pickering emulsion stabilized by surfactant–polymer and nanoparticle–surfactant–polymer systems. Ind. Eng. Chem. Res. 2015, 54, 1576–1584. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, D.; Chen, W.; Liu, B.; Zhang, X. A comprehensive review of emulsion and its field application for enhanced oil recovery. Energy Sci. Eng. 2019, 7, 1046–1058. [Google Scholar] [CrossRef]
- AfzaliTabar, M.; Alaei, M.; Bazmi, M.; Khojasteh, R.R.; Koolivand-Salooki, M.; Motiee, F.; Rashidi, A. Facile and economical preparation method of nanoporous graphene/silica nanohybrid and evaluation of its Pickering emulsion properties for Chemical Enhanced oil Recovery (C-EOR). Fuel 2017, 206, 453–466. [Google Scholar] [CrossRef]
- Son, H.; Kim, H.; Lee, G.; Kim, J.; Sung, W. Enhanced oil recovery using nanoparticle-stabilized oil/water emulsions. Korean J. Chem. Eng. 2014, 31, 338–342. [Google Scholar] [CrossRef]
- Hu, Z.; Nourafkan, E.; Gao, H.; Wen, D. Microemulsions stabilized by in-situ synthesized nanoparticles for enhanced oil recovery. Fuel 2017, 210, 272–281. [Google Scholar] [CrossRef]
- Cheng, H.; Zhou, Y.; Feng, Y.; Geng, W.; Liu, Q.; Guo, W.; Jiang, L. Electrokinetic energy conversion in self-assembled 2D nanofluidic channels with janus nanobuilding blocks. Adv. Mater. 2017, 29, 1700177. [Google Scholar] [CrossRef]
- Polte, J.; Ahner, T.T.; Delissen, F.; Sokolov, S.; Emmerling, F.; Thünemann, A.F.; Kraehnert, R. Mechanism of gold nanoparticle formation in the classical citrate synthesis method derived from coupled in situ XANES and SAXS evaluation. J. Am. Chem. Soc. 2010, 132, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Saien, J.; Akbari, S. Interfacial tension of toluene+ water+ sodium dodecyl sulfate from (20 to 50) C and pH between 4 and 9. J. Chem. Eng. Data 2006, 51, 1832–1835. [Google Scholar] [CrossRef]
- Suslick, K.S. Encyclopedia of physical science and technology. In Sonoluminescence and Sonochemistry, 3rd ed.; Elsevier Science Ltd.: Cambridge, MA, USA, 2001; pp. 1–20. [Google Scholar]
- Nenningsland, A.L.; Simon, S.; Sjoblom, J. Surface properties of basic components extracted from petroleum crude oil. Energy Fuel 2010, 24, 6501–6505. [Google Scholar] [CrossRef]
- Makó, É.; Kovács, A.; Antal, V.; Kristóf, T. One-pot exfoliation of kaolinite by solvothermal cointercalation. Appl. Clay Sci. 2017, 146, 131–139. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Q.; Cheng, H.; Zeng, F. Combined experimental and theoretical investigation of interactions between kaolinite inner surface and intercalated dimethyl sulfoxide. Appl. Surf. Sci. 2015, 331, 234–240. [Google Scholar] [CrossRef]
- Frost, R.; Kristof, J. Raman and infrared spectroscopic studies of kaolinite surfaces modified by intercalation. In Clay Surfaces—Fundamental and Applications; Elsevier: Amsterdam, The Netherlands, 2004; pp. 184–215. [Google Scholar]
- Zhang, Y.; Liu, Q.; Wu, Z.; Zheng, Q.; Cheng, H. Thermal behavior analysis of kaolinite–dimethylsulfoxide intercalation complex. J. Therm. Anal. Calorim. 2012, 110, 1167–1172. [Google Scholar] [CrossRef]
- Zuo, X.; Wang, D.; Zhang, S.; Liu, Q.; Yang, H. Intercalation and exfoliation of kaolinite with sodium dodecyl sulfate. Minerals 2018, 8, 112. [Google Scholar] [CrossRef]
- Ptáček, P.; Kubátová, D.; Havlica, J.; Brandštetr, J.; Šoukal, F.; Opravil, T. Isothermal kinetic analysis of the thermal decomposition of kaolinite: The thermogravimetric study. Thermochim. Acta 2010, 501, 24–29. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, J.; Wang, X.; Wang, Y.; Zhu, J.; Hu, Z. Preparation of KH570-SiO 2 and their modification on the MF/PVA composite membrane. Fiber. Polym. 2015, 16, 1772–1780. [Google Scholar] [CrossRef]
- Chorom, M.; Rengasamy, P. Dispersion and zeta potential of pure clays as related to net particle charge under varying pH, electrolyte concentration and cation type. Eur. J. Soil Sci. 1995, 46, 657–665. [Google Scholar] [CrossRef]
- Yukselen-Aksoy, Y.; Kaya, A. A study of factors affecting on the zeta potential of kaolinite and quartz powder. Environ. Earth Sci. 2011, 62, 697–705. [Google Scholar] [CrossRef]
- Tian, S.; Gao, W.; Liu, Y.; Kang, W.; Yang, H. Effects of surface modification Nano-SiO2 and its combination with surfactant on interfacial tension and emulsion stability. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124682. [Google Scholar] [CrossRef]
- Liang, S.; Li, C.; Dai, L.; Tang, Q.; Cai, X.; Zhen, B.; Xie, X.; Wang, L. Selective modification of kaolinite with vinyltrimethoxysilane for stabilization of Pickering emulsions. Appl. Clay Sci. 2018, 161, 282–289. [Google Scholar] [CrossRef]
- Cai, X.; Li, C.; Tang, Q.; Zhen, B.; Xie, X.; Zhu, W.; Zhou, C.; Wang, L. Assembling kaolinite nanotube at water/oil interface for enhancing Pickering emulsion stability. Appl. Clay Sci. 2019, 172, 115–122. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, K.; Cayre, O.J.; Harbottle, D. Interfacial particle dynamics: One and two step yielding in colloidal glass. Langmuir 2016, 32, 13472–13481. [Google Scholar] [CrossRef]
- Wei, H.-H. Marangoni-enhanced capillary wetting in surfactant-driven superspreading. J. Fluid Mech. 2018, 855, 181–209. [Google Scholar] [CrossRef]
- Cheng, H.-L.; Velankar, S.S. Film climbing of particle-laden interfaces. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 275–284. [Google Scholar] [CrossRef]
- Binks, B.; Clint, J.; Fletcher, P.; Lees, T.; Taylor, P. Growth of gold nanoparticle films driven by the coalescence of particle-stabilized emulsion drops. Langmuir 2006, 22, 4100–4103. [Google Scholar] [CrossRef] [PubMed]
- Shevtsova, T.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Donchak, V.; Harhay, K.; Korolko, S.; Budkowski, A.; Stetsyshyn, Y. Temperature-responsive hybrid nanomaterials based on modified halloysite nanotubes uploaded with silver nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128525. [Google Scholar] [CrossRef]
- Czerniecka-Kubicka, A.; Zarzyka, I.; Schliesser, J.; Woodfield, B.F.; Pyda, M. Vibrational heat capacity of Poly(N-isopropylacrylamide). Polymer 2015, 63, 108–115. [Google Scholar] [CrossRef]
- Czerniecka-Kubicka, A.; Zarzyka, I.; Pyda, M. Long-Term Physical Aging Tracked by Advanced Thermal Analysis of Poly(N-Isopropylacrylamide): A Smart Polymer for Drug Delivery System. Molecules 2020, 25, 3810. [Google Scholar] [CrossRef]
- Wu, H.; Gao, K.; Lu, Y.; Meng, Z.; Gou, C.; Li, Z.; Yang, M.; Qu, M.; Liu, T.; Hou, J. Silica-based amphiphilic Janus nanofluid with improved interfacial properties for enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124162. [Google Scholar] [CrossRef]
- Zhang, L.; Lei, Q.; Luo, J.; Zeng, M.; Wang, L.; Huang, D.; Wang, X.; Mannan, S.; Peng, B.; Cheng, Z. Natural halloysites-based janus platelet surfactants for the formation of pickering emulsion and enhanced oil recovery. Sci. Rep. 2019, 9, 163. [Google Scholar] [CrossRef]
- Wasan, D.T.; Nikolov, A.D. Spreading of nanofluids on solids. Nature 2003, 423, 156–159. [Google Scholar] [CrossRef]
- Kao, R.; Wasan, D.; Nikolov, A.; Edwards, D. Mechanisms of oil removal from a solid surface in the presence of anionic micellar solutions. Colloids Surf. 1988, 34, 389–398. [Google Scholar] [CrossRef]
- Khademolhosseini, R.; Jafari, A.; Shabani, M.H. Micro scale investigation of enhanced oil recovery using nano/bio materials. Procedia Mater. Sci. 2015, 11, 171–175. [Google Scholar] [CrossRef]
- Xue, L.A.G.; Chen, X.; Hao, C. Fluid Flow in Porous Media; World Scientific: Singapore, 2020. [Google Scholar] [CrossRef]
- Guo, H.; Dou, M.; Hanqing, W.; Wang, F.; Yuanyuan, G.; Yu, Z.; Yansheng, W.; Li, Y. Proper use of capillary number in chemical flooding. J. Chem. 2017, 2017, 4307368. [Google Scholar] [CrossRef]
- Mohyaldinn, M.E.; Hassan, A.M.; Ayoub, M.A. Application of emulsions and microemulsions in enhanced oil recovery and well stimulation. In Microemulsion-a Chemical Nanoreactor; IntechOpen: London, UK, 2019. [Google Scholar]
- Hirose, Y.; Komura, S.; Nonomura, Y. Adsorption of Janus particles to curved interfaces. J. Chem. Phys. 2007, 127, 054707. [Google Scholar] [CrossRef]













| Core | Core Permeability (mD) | Water Flooding Recovery Rate (%) | Nanofluid Flooding Recovery Rate (%) | Total Recovery Rate (%) |
|---|---|---|---|---|
| Kaol (#1) | 496 | 33.57 | 13.18 | 46.75 |
| KaolNS (#2) | 490 | 38.97 | 12.98 | 51.95 |
| KaolKH@40 (#3) | 470 | 39.47 | 22.37 | 61.84 |
| KaolKH@70 (#4) | 450 | 44.3 | 12.67 | 56.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Y.; Lanzon, A.L.; Zheng, B.; Xu, Z.; Jiang, J.; Harbottle, D.; Yu, K.; Chen, M.; Sheng, Y.; Zhang, H. Nanofluids of Amphiphilic Kaolinite-Based Janus Nanosheets for Enhanced Oil Recovery: The Importance of Stable Emulsion. Polymers 2023, 15, 2515. https://doi.org/10.3390/polym15112515
Mao Y, Lanzon AL, Zheng B, Xu Z, Jiang J, Harbottle D, Yu K, Chen M, Sheng Y, Zhang H. Nanofluids of Amphiphilic Kaolinite-Based Janus Nanosheets for Enhanced Oil Recovery: The Importance of Stable Emulsion. Polymers. 2023; 15(11):2515. https://doi.org/10.3390/polym15112515
Chicago/Turabian StyleMao, Yixuan, Alain Luigi Lanzon, Botuo Zheng, Zhengxiao Xu, Jiatong Jiang, David Harbottle, Kai Yu, Mingfeng Chen, Yu Sheng, and Huagui Zhang. 2023. "Nanofluids of Amphiphilic Kaolinite-Based Janus Nanosheets for Enhanced Oil Recovery: The Importance of Stable Emulsion" Polymers 15, no. 11: 2515. https://doi.org/10.3390/polym15112515
APA StyleMao, Y., Lanzon, A. L., Zheng, B., Xu, Z., Jiang, J., Harbottle, D., Yu, K., Chen, M., Sheng, Y., & Zhang, H. (2023). Nanofluids of Amphiphilic Kaolinite-Based Janus Nanosheets for Enhanced Oil Recovery: The Importance of Stable Emulsion. Polymers, 15(11), 2515. https://doi.org/10.3390/polym15112515