Effects of Propolis Impregnation on Polylactic Acid (PLA) Scaffolds Loaded with Wollastonite Particles against Staphylococcus aureus, Staphylococcus epidermidis, and Their Coculture for Potential Medical Devices
Abstract
:1. Introduction
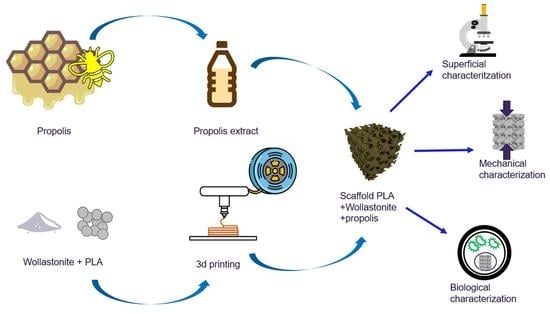
2. Materials and Methods
2.1. Filament Fabrication
2.2. Collection and Characterization of Propolis Extracts
2.3. Scaffolds Fabrication
2.4. Scaffolds Characterization
2.4.1. Scanning Electron Microscopy
2.4.2. Contact Angle
2.4.3. Swelling and Degradation Tests
2.4.4. Fourier-Transformed Infrared Spectroscopy
2.4.5. Differential Scanning Calorimetry
2.4.6. Mechanical Response
- Monotonic static tests. Monotonic static compression tests on the scaffolds were conducted using a universal testing machine (Instron 3366, Instron, MA, USA) under a static compression configuration using a force sensor with a maximum range of 3 kN at a speed of 2 mm/min. Force and displacement values were acquired during the test. Five samples for each condition were tested.
- Cyclic tests. Cyclic compressive tests were performed on the scaffolds using a sinusoidal wave of 1 Hz frequency to follow the typical masticatory frequency by employing dynamic testing equipment (TA Electro Force 300) with a load cell of 500 N maximum capacity. Tests were conducted at two different cyclic conditions, depending on the expected maximum load values. First, tests with a maximum load Fmax = 450 N and a minimum load Fmin = 45 N, corresponding to an R of 0.1, were performed for up to 8000 cycles to find the maximum deformation in the scaffold. This test condition is considered representative of high masticatory loads, considering that the reported maximum masticatory loads for single occlusions reach up to 1200 N. Moreover, tests with Fmax = 45 N and Fmin = 4.5 N were conducted for up to 500 × 103 cycles to get a condition similar to typical masticatory loads during prolonged periods. For each condition, at least five samples were tested.
2.4.7. Antibacterial Activity
- Bacterial strains. Antibacterial activity was assessed against the following standard strains: S. aureus (ATCC 25175) and S. epidermidis (ATCC 12228). The strains were previously seeded in Petri dishes containing Mueller–Hinton agar (Merck, Darmstadt, Germany) and incubated at 37 °C for 24 h. To obtain bacterial inocula, the strains were grown to an exponential phase in a brain heart infusion medium (Merck, Germany) at 37 °C for 24 h and adjusted by diluting the new cultures until reaching a turbidity equivalent to 90 NTU (approximately 1.5 × 108 CFU/mL).
- Determination of the minimum inhibitory concentration (MIC). The MIC was tested for both strains. For this, the pure extract was taken, and serial dilutions were made by taking EEP concentrations ranging from 10% to 0.01% against S. aureus and S. epidermidis. The obtained inoculum at approximately 1.5 × 105 CFU/mL was then assessed. The inoculum was incubated for 24 h at 37 °C and 5% CO2 at different EEP concentrations. The MIC was determined as the final concentration without a visible growth of bacteria.
- Inhibition zone tests. We used a 90 NTU (approximately 1.5 × 108 CFU/mL) inoculum and the disk diffusion method to assess the antibacterial activity. Twenty microliters of Mueller–Hinton agar medium was poured onto Petri dishes. Each Petri dish was inoculated with a bacterial inoculum. Next, the scaffolds were placed and divided into two groups: the first was impregnated with propolis extracts (150 mg/mL) (CS), and the second was not (US). 0.2% chlorhexidine digluconate and PBS were used as positive and negative control agents. After 24 h of incubation at 37 °C, the inhibition zone was measured in mm using ImageJ; each experiment was performed three times.
- Biofilm-formation assays for S. aureus, S. epidermidis, and their coculture. The adhesion assay was performed with each strain and their coculture. 90 NTU (approximately 1.5 × 108 CFU/mL) inocula were used to create two 1:10 dilutions until reaching a 1.5 × 106 CFU/mL concentration for each strain. To prepare the coculture, the two strains were mixed at a concentration of 1.5 × 106 CFU/mL in equal parts. Then, previously sterilized scaffolds were taken, and two groups were formed: the first was impregnated with propolis extracts (CS) (150 mg/mL), and the second was not (US). Both groups were seeded with 1 mL inoculum from each strain and their coculture. The scaffolds were incubated for 24 h and 48 h at 37 °C and 5% CO2. After incubation, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT, Merck, Germany) was added and incubated for 2 h at 37 °C and 5% CO2. The MTT was then removed, and dimethylsulfoxide (Sigma, USA) was added. Finally, a spectrophotometer (Zeiss, Oberkochen, Germany) was used to measure the absorbance at 550 nm.
2.4.8. Cell Viability/Proliferation Assay
2.4.9. Statistical Analysis
3. Results and Discussion
3.1. Wollastonite Particles, Filaments, and Scaffolds
3.2. Scaffold Characterization: Hydrophobicity, Swelling, and Degradation
3.3. Fourier-Transform Infrared Spectroscopy
3.4. Thermal Behavior
3.5. Mechanical Behavior
3.6. Biological Characterization
3.6.1. Propolis Extracts
Total Phenols and Flavonoids
Antibacterial Activity of Propolis Extracts
3.6.2. Zone of Inhibition Assays with Propolis-Loaded Scaffolds
Viability of Bacterial Biofilms on Scaffolds
Cell Viability/Proliferation Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ba, X.; Hadjiargyrou, M.; Di Masi, E.; Meng, Y.; Simon, M.; Tan, Z.; Rafailovich, M.H. The Role of Moderate Static Magnetic Fields on Biomineralization of Osteoblasts on Sulfonated Polystyrene Films. Biomaterials 2011, 32, 7831–7838. [Google Scholar] [CrossRef] [PubMed]
- Martin-Piedra, A.; Martin-Piedra, L. Matrices para Ingeniería del tejido óseo. Actual. Med. 2019, 104, 36–45. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrivats, A.R.; McDermott, M.C.; Hollinger, J.O. Bone Tissue Engineering: State of the Union. Drug Discov. Today 2014, 19, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Carletti, E.; Motta, A.; Migliaresi, C. Scaffolds for Tissue Engineering and 3D Cell Culture. In 3D Cell Culture; Haycock, J.W., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 695, pp. 17–39. ISBN 978-1-60761-983-3. [Google Scholar]
- Saleh Alghamdi, S.; John, S.; Roy Choudhury, N.; Dutta, N.K. Additive Manufacturing of Polymer Materials: Progress, Promise and Challenges. Polymers 2021, 13, 753. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D Printing of Ceramics: A Review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Jorge, L.S.; Chueire, A.G.; Baptista Rossit, A.R. Osteomyelitis: A Current Challenge. Braz. J. Infect. Dis. 2010, 14, 310–315. [Google Scholar] [CrossRef] [Green Version]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for Bone Tissue Engineering Scaffolds: A Review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [Green Version]
- Abudhahir, M.; Saleem, A.; Paramita, P.; Kumar, S.D.; Tze-Wen, C.; Selvamurugan, N.; Moorthi, A. Polycaprolactone Fibrous Electrospun Scaffolds Reinforced with Copper Doped Wollastonite for Bone Tissue Engineering Applications. J. Biomed. Mater. Res. 2021, 109, 654–664. [Google Scholar] [CrossRef]
- Liu, A.; Sun, M.; Shao, H.; Yang, X.; Ma, C.; He, D.; Gao, Q.; Liu, Y.; Yan, S.; Xu, S.; et al. The Outstanding Mechanical Response and Bone Regeneration Capacity of Robocast Dilute Magnesium-Doped Wollastonite Scaffolds in Critical Size Bone Defects. J. Mater. Chem. B 2016, 4, 3945–3958. [Google Scholar] [CrossRef]
- Shao, H.; Liu, A.; Ke, X.; Sun, M.; He, Y.; Yang, X.; Fu, J.; Zhang, L.; Yang, G.; Liu, Y.; et al. 3D Robocasting Magnesium-Doped Wollastonite/TCP Bioceramic Scaffolds with Improved Bone Regeneration Capacity in Critical Sized Calvarial Defects. J. Mater. Chem. B 2017, 5, 2941–2951. [Google Scholar] [CrossRef]
- Lee, J.W.; Ahn, G.; Kim, D.S.; Cho, D.-W. Development of Nano- and Microscale Composite 3D Scaffolds Using PPF/DEF-HA and Micro-Stereolithography. Microelectron. Eng. 2009, 86, 1465–1467. [Google Scholar] [CrossRef]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly (lactic acid)-Based biomaterials for orthopaedic regenerative engineering. Adv. Drug Deliv. Rev. 2016, 107, 247–276. [Google Scholar] [CrossRef] [Green Version]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic Acid: Synthesis and Biomedical Applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Gao, C.; Jiang, J.; Wu, Y.; Zhu, P.; Chen, G. 3D Printed Porous PLA/NHA Composite Scaffolds with Enhanced Osteogenesis and Osteoconductivity in Vivo for Bone Regeneration. Biomed. Mater. 2019, 14, 065003. [Google Scholar] [CrossRef]
- Sawyer, A.A.; Song, S.J.; Susanto, E.; Chuan, P.; Lam, C.X.F.; Woodruff, M.A.; Hutmacher, D.W.; Cool, S.M. The Stimulation of Healing within a Rat Calvarial Defect by MPCL–TCP/Collagen Scaffolds Loaded with RhBMP-2. Biomaterials 2009, 30, 2479–2488. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Liang, H.; Gao, C.; Peng, S.; Shen, L.; Shuai, C. Additive Manufacturing of Bone Scaffolds. Int. J. Bioprint. 2018, 5, 148. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Gervaso, F.; Scalera, F.; Padmanabhan, S.K.; Madaghiele, M.; Montagna, F.; Sannino, A.; Licciulli, A.; Maffezzoli, A. Highly Loaded Hydroxyapatite Microsphere/PLA Porous Scaffolds Obtained by Fused Deposition Modelling. Ceram. Int. 2019, 45, 2803–2810. [Google Scholar] [CrossRef]
- Zein, I.; Hutmacher, D.W.; Tan, K.C.; Teoh, S.H. Fused Deposition Modeling of Novel Scaffold Architectures for Tissue Engineering Applications. Biomaterials 2002, 23, 1169–1185. [Google Scholar] [CrossRef]
- Leong, K.F.; Cheah, C.M.; Chua, C.K. Solid Freeform Fabrication of Three-Dimensional Scaffolds for Engineering Replacement Tissues and Organs. Biomaterials 2003, 24, 2363–2378. [Google Scholar] [CrossRef]
- Dong, Z.; Zhao, X. Application of TPMS Structure in Bone Regeneration. Eng. Regen. 2021, 2, 154–162. [Google Scholar] [CrossRef]
- Lu, F.; Wu, R.; Shen, M.; Xie, L.; Liu, M.; Li, Y.; Xu, S.; Wan, L.; Yang, X.; Gao, C.; et al. Rational Design of Bioceramic Scaffolds with Tuning Pore Geometry by Stereolithography: Microstructure Evaluation and Mechanical Evolution. J. Eur. Ceram. Soc. 2021, 41, 1672–1682. [Google Scholar] [CrossRef]
- Restrepo, S.; Ocampo, S.; Ramírez, J.A.; Paucar, C.; García, C. Mechanical Properties of Ceramic Structures Based on Triply Periodic Minimal Surface (TPMS) Processed by 3D Printing. J. Phys. Conf. Ser. 2017, 935, 012036. [Google Scholar] [CrossRef] [Green Version]
- Al-Ketan, O.; Abu Al-Rub, R.K. Multifunctional Mechanical Metamaterials Based on Triply Periodic Minimal Surface Lattices. Adv. Eng. Mater. 2019, 21, 1900524. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.; Borba, R.; Wilson, M.; Spivak, M. Propolis Counteracts Some Threats to Honey Bee Health. Insects 2017, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Becerra, T.B.; Calla-Poma, R.D.; Requena-Mendizabal, M.F.; Millones-Gómez, P.A. Antibacterial Effect of Peruvian Propolis Collected During Different Seasons on the Growth of Streptococcus Mutans. TODENTJ 2019, 13, 327–331. [Google Scholar] [CrossRef]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent Progress of Propolis for Its Biological and Chemical Compositions and Its Botanical Origin. Evid.-Based Complement. Altern. Med. 2013, 2013, 697390. [Google Scholar] [CrossRef]
- Afrouzan, H.; Tahghighi, A.; Zakeri, S.; Es-haghi, A. Chemical Composition and Antimicrobial Activities of Iranian Propolis. IBJ 2018, 22, 50–65. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Potential Role of Propolis in Wound Healing: Biological Properties and Therapeutic Activities. Biomed. Pharmacother. 2018, 98, 469–483. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.A.; Abd El-Aziz, S.; Elbadry, H.M.; El-Aassar, S.A.; Tamer, T.M. Prevalence, Antimicrobial Resistance Profile, and Characterization of Multi-Drug Resistant Bacteria from Various Infected Wounds in North Egypt. Saudi J. Biol. Sci. 2022, 29, 2978–2988. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [Green Version]
- Demir, S.; Aliyazicioglu, Y.; Turan, I.; Misir, S.; Mentese, A.; Yaman, S.O.; Akbulut, K.; Kilinc, K.; Deger, O. Antiproliferative and Proapoptotic Activity of Turkish Propolis on Human Lung Cancer Cell Line. Nutr. Cancer 2016, 68, 165–172. [Google Scholar] [CrossRef]
- Orsi, R.O.; Fernandes, A.; Bankova, V.; Sforcin, J.M. The Effects of Brazilian and Bulgarian Propolis in Vitro against Salmonella Typhi and Their Synergism with Antibiotics Acting on the Ribosome. Nat. Prod. Res. 2012, 26, 430–437. [Google Scholar] [CrossRef]
- Ceylan, O.; Karakus, H.; Cicek, H. Design and In Vitro Antibiofilm Activity of Propolis Diffusion-Controlled Biopolymers. Biotechnol. Appl. Biochem. 2021, 68, 789–800. [Google Scholar] [CrossRef]
- Nori, M.P.; Favaro-Trindade, C.S.; Matias de Alencar, S.; Thomazini, M.; de Camargo Balieiro, J.C.; Contreras Castillo, C.J. Microencapsulation of Propolis Extract by Complex Coacervation. LWT Food Sci. Technol. 2011, 44, 429–435. [Google Scholar] [CrossRef]
- Sanpa, S.; Sutjarittangtham, K.; Tunkasiri, T.; Eitssayeam, S.; Chantawannakul, P. Antimicrobial Effect of Brazillian Propolis/Polycaprolactone Polymer on Some Human Pathogenic Bacteria. AMR 2012, 506, 537–540. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Doostan, M.; Khorramizadeh, M.R. Antibacterial and Antioxidant Assessment of Cellulose Acetate/Polycaprolactone Nanofibrous Mats Impregnated with Propolis. Int. J. Biol. Macromol. 2019, 140, 1260–1268. [Google Scholar] [CrossRef]
- da Costa Silva, V.; do Nascimento, T.G.; Mergulhão, N.L.O.N.; Freitas, J.D.; Duarte, I.F.B.; de Bulhões, L.C.G.; Dornelas, C.B.; de Araújo, J.X.; dos Santos, J.; Silva, A.C.A.; et al. Development of a Polymeric Membrane Impregnated with Poly-Lactic Acid (PLA) Nanoparticles Loaded with Red Propolis (RP). Molecules 2022, 27, 6959. [Google Scholar] [CrossRef]
- González-Masís, J.; Cubero-Sesin, J.M.; Corrales-Ureña, Y.R.; González-Camacho, S.; Mora-Ugalde, N.; Baizán-Rojas, M.; Loaiza, R.; Vega-Baudrit, J.R.; Gonzalez-Paz, R.J. Increased Fibroblast Metabolic Activity of Collagen Scaffolds via the Addition of Propolis Nanoparticles. Materials 2020, 13, 3118. [Google Scholar] [CrossRef]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant Activity of Propolis of Various Geographic Origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Rahmani, P.; Shojaei, A. Developing Tough Terpolymer Hydrogel with Outstanding Swelling Ability by Hydrophobic Association Cross-Linking. Polymer 2022, 254, 125037. [Google Scholar] [CrossRef]
- Sadeghianmaryan, A.; Naghieh, S.; Yazdanpanah, Z.; Alizadeh Sardroud, H.; Sharma, N.K.; Wilson, L.D.; Chen, X. Fabrication of Chitosan/Alginate/Hydroxyapatite Hybrid Scaffolds Using 3D Printing and Impregnating Techniques for Potential Cartilage Regeneration. Int. J. Biol. Macromol. 2022, 204, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Li, M.G.; Cooper, D.; Chen, X.B. Development of Novel Hybrid Poly(L-lactide)/Chitosan Scaffolds Using the Rapid Freeze Prototyping Technique. Biofabrication 2011, 3, 034105. [Google Scholar] [CrossRef] [PubMed]
- Bidan, C.M.; Kommareddy, K.P.; Rumpler, M.; Kollmannsberger, P.; Fratzl, P.; Dunlop, J.W.C. Geometry as a Factor for Tissue Growth: Towards Shape Optimization of Tissue Engineering Scaffolds. Adv. Healthc. Mater. 2013, 2, 186–194. [Google Scholar] [CrossRef]
- Garcia, C.; Orozco, Y.; Betancur, A.; Moreno, A.I.; Fuentes, K.; Lopera, A.; Suarez, O.; Lobo, T.; Ossa, A.; Peláez-Vargas, A.; et al. Fabrication of Polycaprolactone/Calcium Phosphates Hybrid Scaffolds Impregnated with Plant Extracts Using 3D Printing for Potential Bone Regeneration. Heliyon 2023, 9, e13176. [Google Scholar] [CrossRef]
- Ramírez, J.A.; Ospina, V.; Rozo, A.A.; Viana, M.I.; Ocampo, S.; Restrepo, S.; Vásquez, N.A.; Paucar, C.; García, C. Influence of Geometry on Cell Proliferation of PLA and Alumina Scaffolds Constructed by Additive Manufacturing. J. Mater. Res. 2019, 34, 3757–3765. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Lopera, A.A.; Montoya, A.; Vélez, I.D.; Robledo, S.M.; Garcia, C.P. Synthesis of Calcium Phosphate Nanostructures by Combustion in Solution as a Potential Encapsulant System of Drugs with Photodynamic Properties for the Treatment of Cutaneous Leishmaniasis. Photodiagn. Photodyn. Ther. 2018, 21, 138–146. [Google Scholar] [CrossRef]
- Nevado, P.; Lopera, A.; Bezzon, V.; Fulla, M.R.; Palacio, J.; Zaghete, M.A.; Biasotto, G.; Montoya, A.; Rivera, J.; Robledo, S.M.; et al. Preparation and In Vitro Evaluation of PLA/Biphasic Calcium Phosphate Filaments Used for Fused Deposition Modelling of Scaffolds. Mater. Sci. Eng. C 2020, 114, 111013. [Google Scholar] [CrossRef]
- Mendoza, E.; Garcia, C. Sol-Gel Coatings Containing Wollastonite Particles on Stainless Steel 316L. Sci. Technol. 2007, 36, 413–417. [Google Scholar]
- Sedelnikova, M.B.; Ugodchikova, A.V.; Tolkacheva, T.V.; Chebodaeva, V.V.; Cluklhov, I.A.; Khimich, M.A.; Bakina, O.V.; Lerner, M.I.; Egorkin, V.S.; Schmidt, J.; et al. Surface Modification of Mg0.8Ca Alloy via Wollastonite Micro-Arc Coatings: Significant Improvement in Corrosion Resistance. Metals 2021, 11, 754. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, C.; Zhou, S.; Luo, C. The Self-Gelation Properties of Calcined Wollastonite Powder. Constr. Build. Mater. 2021, 290, 123061. [Google Scholar] [CrossRef]
- Kalinkina, E.V.; Kalinkin, A.M.; Forsling, W.; Makarov, V.N. Sorption of Atmospheric Carbon Dioxide and Structural Changes of Ca and Mg Silicate Minerals during Grinding. Int. J. Miner. Process. 2001, 61, 273–288. [Google Scholar] [CrossRef]
- Cai, R.; Wang, S.; Meng, Y.; Meng, Q.; Zhao, W. Rapid Quantification of Flavonoids in Propolis and Previous Study for Classification of Propolis from Different Origins by Using near Infrared Spectroscopy. Anal. Methods 2012, 4, 2388–2395. [Google Scholar] [CrossRef]
- Svečnjak, L.; Marijanović, Z.; Okińczyc, P.; Marek Kuś, P.; Jerković, I. Mediterranean Propolis from the Adriatic Sea Islands as a Source of Natural Antioxidants: Comprehensive Chemical Biodiversity Determined by GC-MS, FTIR-ATR, UHPLC-DAD-QqTOF-MS, DPPH and FRAP Assay. Antioxidants 2020, 9, 337. [Google Scholar] [CrossRef] [Green Version]
- Banskota, A.H.; Tezuka, Y.; Kadota, S. Recent Progress in Pharmacological Research of Propolis. Phytother. Res. 2001, 15, 561–571. [Google Scholar] [CrossRef]
- Byun, Y.; Rodriguez, K.; Han, J.H.; Kim, Y.T. Improved Thermal Stability of Polylactic Acid (PLA) Composite Film via PLA–β-Cyclodextrin-Inclusion Complex Systems. Int. J. Biol. Macromol. 2015, 81, 591–598. [Google Scholar] [CrossRef]
- Byun, Y.; Whiteside, S.; Thomas, R.; Dharman, M.; Hughes, J.; Kim, Y.T. The Effect of Solvent Mixture on the Properties of Solvent Cast Polylactic Acid (PLA) Film. J. Appl. Polym. Sci. 2012, 124, 3577–3582. [Google Scholar] [CrossRef]
- Marșavina, L.; Vălean, C.; Mărghitaș, M.; Linul, E.; Razavi, S.M.J.; Berto, F.; Brighenti, R. Effect of the Manufacturing Parameters on the Tensile and Fracture Properties of FDM 3D-Printed PLA Specimens. Eng. Fract. Mech. 2022, 274, 108766. [Google Scholar] [CrossRef]
- Sahinler, N.; Kaftanoglu, O. Natural Product Propolis: Chemical Composition. Nat. Prod. Res. 2005, 19, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.K.S.; Denadai, M.; de Oliveira, C.S.; Nunes, M.L.; Narain, N. Evaluation of Bioactive Compounds Potential and Antioxidant Activity of Brown, Green and Red Propolis from Brazilian Northeast Region. Food Res. Int. 2017, 101, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Christov, R.; Trusheva, B.; Popova, M.; Bankova, V.; Bertrand, M. Chemical Composition of Propolis from Canada, Its Antiradical Activity and Plant Origin. Nat. Prod. Res. 2006, 20, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Almeida, M.O.; Lemos, M.; Arruda, C.; Casoti, R.; Somensi, L.B.; Boeing, T.; Mariott, M.; da Silva, R.d.C.M.V.d.A.F.; Stein, B.D.P.; et al. Artepillin C, Drupanin, Aromadendrin-4′-O-Methyl-Ether and Kaempferide from Brazilian Green Propolis Promote Gastroprotective Action by Diversified Mode of Action. J. Ethnopharmacol. 2018, 226, 82–89. [Google Scholar] [CrossRef]
- Kudo, D.; Inden, M.; Sekine, S.; Tamaoki, N.; Iida, K.; Naito, E.; Watanabe, K.; Kamishina, H.; Shibata, T.; Hozumi, I. Conditioned Medium of Dental Pulp Cells Stimulated by Chinese Propolis Show Neuroprotection and Neurite Extension In Vitro. Neurosci. Lett. 2015, 589, 92–97. [Google Scholar] [CrossRef]
- Alshaher, A.; Wallace, J.; Agarwal, S.; Bretz, W.; Baugh, D. Effect of Propolis on Human Fibroblasts from the Pulp and Periodontal Ligament. J. Endod. 2004, 30, 359–361. [Google Scholar] [CrossRef]
- Burdock, G.A. Review of the Biological Properties and Toxicity of Bee Propolis (Propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Cottica, S.M.; Sawaya, A.C.H.F.; Eberlin, M.N.; Franco, S.L.; Zeoula, L.M.; Visentainer, J.V. Antioxidant Activity and Composition of Propolis Obtained by Different Methods of Extraction. J. Braz. Chem. Soc. 2011, 22, 929–935. [Google Scholar] [CrossRef]
- Pobiega, K.; Kraśniewska, K.; Derewiaka, D.; Gniewosz, M. Comparison of the Antimicrobial Activity of Propolis Extracts Obtained by Means of Various Extraction Methods. J. Food Sci. Technol. 2019, 56, 5386–5395. [Google Scholar] [CrossRef] [Green Version]
- Trusheva, B.; Trunkova, D.; Bankova, V. Different Extraction Methods of Biologically Active Components from Propolis: A Preliminary Study. Chem. Cent. J. 2007, 1, 13. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, M.; Modarai, M.; Kortenkamp, A. Herbal Extracts Used for Upper Respiratory Tract Infections: Are There Clinically Relevant Interactions with the Cytochrome P450 Enzyme System? Planta Med. 2008, 74, 657–660. [Google Scholar] [CrossRef]
- Moussaoui, S.; Lahouel, M. Propolis Extract: A Potent Bacteria Efflux Pump Inhibitor. J. Biol. Act. Prod. Nat. 2014, 4, 216–223. [Google Scholar] [CrossRef]
- Garzoli, S.; Maggio, F.; Vinciguerra, V.; Rossi, C.; Donadu, M.G.; Serio, A. Chemical Characterization and Antimicrobial Properties of the Hydroalcoholic Solution of Echinacea purpurea (L.) Moench. and Propolis from Northern Italy. Molecules 2023, 28, 1380. [Google Scholar] [CrossRef]
- Gonçalves, I.S.; Lima, L.R.; Berretta, A.A.; Amorim, N.A.; Pratavieira, S.; Corrêa, T.Q.; Nogueira, F.A.R.; Barud, H.S. Antimicrobial Formulation of a Bacterial Nanocellulose/Propolis-Containing Photosensitizer for Biomedical Applications. Polymers 2023, 15, 987. [Google Scholar] [CrossRef]
- Gonzalez-Pastor, R.; Carrera-Pacheco, S.E.; Zúñiga-Miranda, J.; Rodríguez-Pólit, C.; Mayorga-Ramos, A.; Guamán, L.P.; Barba-Ostria, C. Current Landscape of Methods to Evaluate Antimicrobial Activity of Natural Extracts. Molecules 2023, 28, 1068. [Google Scholar] [CrossRef]
- Tamer, T.M.; Alsehli, M.H.; Omer, A.M.; Afifi, T.H.; Sabet, M.M.; Mohy-Eldin, M.S.; Hassan, M.A. Development of Polyvinyl Alcohol/Kaolin Sponges Stimulated by Marjoram as Hemostatic, Antibacterial, and Antioxidant Dressings for Wound Healing Promotion. Int. J. Mol. Sci. 2021, 22, 13050. [Google Scholar] [CrossRef]
- da Cruz Almeida, E.T.; da Silva, M.C.; dos Santos Oliveira, J.M.; Kamiya, R.U.; dos Santos Arruda, R.E.; Vieira, D.A.; da Costa Silva, V.; Escodro, P.B.; Basílio-Júnior, I.D.; do Nascimento, T.G. Chemical and Microbiological Characterization of Tinctures and Microcapsules Loaded with Brazilian Red Propolis Extract. J. Pharm. Anal. 2017, 7, 280–287. [Google Scholar] [CrossRef]
- Carek, P.J.; Dickerson, L.M.; Sackier, J.M. Diagnosis and management of osteomyelitis. Am. Fam. Physician 2001, 63, 2413–2420. [Google Scholar]
- Peschel, A.; Otto, M. Phenol-Soluble Modulins and Staphylococcal Infection. Nat. Rev. Microbiol. 2013, 11, 667–673. [Google Scholar] [CrossRef]
- Gonsales, G.Z.; Orsi, R.O.; Fernandes Júnior, A.; Rodrigues, P.; Funari, S.R.C. Antibacterial Activity of Propolis Collected in Different Regions of Brazil. J. Venom. Anim. Toxins Incl. Trop. Dis. 2006, 12, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Richardson, A.; Sofian-Azirun, M. Antibacterial activity of propolis and honey against Staphylococcus aureus and Escherichia coli. Afr. J. Microbiol. Res. 2010, 4, 1872–1878. [Google Scholar]
- Massuda, K.F. Parâmetros Físico-Químicos e Atividade Biológica da Própolis Submetida a Diferentes Tipos de Extração. Ph.D. Thesis, Universidade Estadual Paulista, Rio Claro, Brazil, 2003. [Google Scholar]
- Lu, L.-C.; Chen, Y.-W.; Chou, C.-C. Antibacterial Activity of Propolis against Staphylococcus Aureus. Int. J. Food Microbiol. 2005, 102, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Abd Rashid, N.; Mohammed, S.N.F.; Syed Abd Halim, S.A.; Ghafar, N.A.; Abdul Jalil, N.A. Therapeutic Potential of Honey and Propolis on Ocular Disease. Pharmaceuticals 2022, 15, 1419. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef] [Green Version]
- Seidel, V.; Peyfoon, E.; Watson, D.G.; Fearnley, J. Comparative Study of the Antibacterial Activity of Propolis from Different Geographical and Climatic Zones: Antibacterial Activity of Propolis from Different Zones. Phytother. Res. 2008, 22, 1256–1263. [Google Scholar] [CrossRef]
- Periasamy, S.; Chatterjee, S.S.; Cheung, G.Y.C.; Otto, M. Phenol-Soluble Modulins in Staphylococci: What Are They Originally For? Commun. Integr. Biol. 2012, 5, 275–277. [Google Scholar] [CrossRef]
- Cheung, G.Y.C.; Joo, H.-S.; Chatterjee, S.S.; Otto, M. Phenol-Soluble Modulins—Critical Determinants of Staphylococcal Virulence. FEMS Microbiol. Rev. 2014, 38, 698–719. [Google Scholar] [CrossRef] [Green Version]
- Shehata, M.G.; Ahmad, F.T.; Badr, A.N.; Masry, S.H.; El-Sohaimy, S.A. Chemical Analysis, Antioxidant, Cytotoxic and Antimicrobial Properties of Propolis from Different Geographic Regions. Ann. Agric. Sci. 2020, 65, 209–217. [Google Scholar] [CrossRef]
- Tatlısulu, S.; Özgör, E. Identification of Cyprus Propolis Composition and Evaluation of Its Antimicrobial and Antiproliferative Activities. Food Biosci. 2023, 51, 102273. [Google Scholar] [CrossRef]
- Seda Vatansever, H.; Sorkun, K.; İsmet Deliloğlu Gurhan, S.; Ozdal-Kurt, F.; Turkoz, E.; Gencay, O.; Salih, B. Propolis from Turkey Induces Apoptosis through Activating Caspases in Human Breast Carcinoma Cell Lines. Acta Histochem. 2010, 112, 546–556. [Google Scholar] [CrossRef]
- Choudhari, M.K.; Haghniaz, R.; Rajwade, J.M.; Paknikar, K.M. Anticancer Activity of Indian Stingless Bee Propolis: An In Vitro Study. Evid.-Based Complement. Altern. Med. 2013, 2013, 928280. [Google Scholar] [CrossRef] [Green Version]
- Salehi, A.; Rezaei, A.; Damavandi, M.S.; Kharazmi, M.S.; Jafari, S.M. Almond Gum-Sodium Caseinate Complexes for Loading Propolis Extract: Characterization, Antibacterial Activity, Release, and In-Vitro Cytotoxicity. Food Chem. 2023, 405, 134801. [Google Scholar] [CrossRef]










| Material | Melting Temperature | Fusion Enthalphy (ΔHexp) J/g | Cristalinity Index (CI) |
|---|---|---|---|
| PLA Raw Material | 115.03 | 79.185 | 74.703 |
| PLA Filament | 116.59 | 71.948 | 97.875 |
| PLA + W particles filament | 116.64 | 46.572 | 54.919 |
| PLA Scaffolds | 116.67 | 68.322 | 64.454 |
| PLA + W Particles Scaffolds | 115.39 | 54.449 | 64.209 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, A.I.; Orozco, Y.; Ocampo, S.; Malagón, S.; Ossa, A.; Peláez-Vargas, A.; Paucar, C.; Lopera, A.; Garcia, C. Effects of Propolis Impregnation on Polylactic Acid (PLA) Scaffolds Loaded with Wollastonite Particles against Staphylococcus aureus, Staphylococcus epidermidis, and Their Coculture for Potential Medical Devices. Polymers 2023, 15, 2629. https://doi.org/10.3390/polym15122629
Moreno AI, Orozco Y, Ocampo S, Malagón S, Ossa A, Peláez-Vargas A, Paucar C, Lopera A, Garcia C. Effects of Propolis Impregnation on Polylactic Acid (PLA) Scaffolds Loaded with Wollastonite Particles against Staphylococcus aureus, Staphylococcus epidermidis, and Their Coculture for Potential Medical Devices. Polymers. 2023; 15(12):2629. https://doi.org/10.3390/polym15122629
Chicago/Turabian StyleMoreno, Ana Isabel, Yeison Orozco, Sebastián Ocampo, Sarita Malagón, Alex Ossa, Alejandro Peláez-Vargas, Carlos Paucar, Alex Lopera, and Claudia Garcia. 2023. "Effects of Propolis Impregnation on Polylactic Acid (PLA) Scaffolds Loaded with Wollastonite Particles against Staphylococcus aureus, Staphylococcus epidermidis, and Their Coculture for Potential Medical Devices" Polymers 15, no. 12: 2629. https://doi.org/10.3390/polym15122629
APA StyleMoreno, A. I., Orozco, Y., Ocampo, S., Malagón, S., Ossa, A., Peláez-Vargas, A., Paucar, C., Lopera, A., & Garcia, C. (2023). Effects of Propolis Impregnation on Polylactic Acid (PLA) Scaffolds Loaded with Wollastonite Particles against Staphylococcus aureus, Staphylococcus epidermidis, and Their Coculture for Potential Medical Devices. Polymers, 15(12), 2629. https://doi.org/10.3390/polym15122629