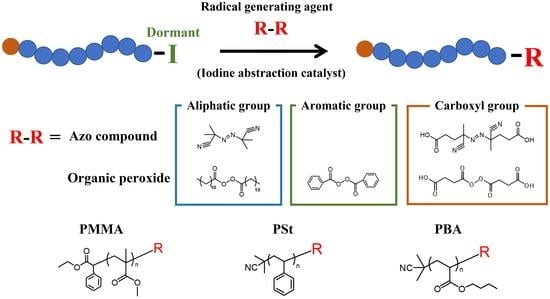
Novel Chain-End Modification of Polymer Iodides via Reversible Complexation-Mediated Polymerization with Functionalized Radical Generation Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polymer-I Precursor via RCMP
2.3. Chain-End Modification of Polymer-I
2.4. Experiment to Estimate the Combination Rate Constant kc
2.5. Characterization
3. Results and Discussion
3.1. PMMA-I Modification
3.1.1. Effect of Chemical Structure of Radical Generating Agent with or without Iodine Abstraction Catalyst on Chain-End Modification Efficiency
Effect of Chemical Structure of Radical Generating Agent without Iodine Abstraction Catalyst on Chain-End Modification Efficiency
Effect of Addition of Iodine Abstraction Catalyst on Chain-End Modification Efficiency
Effect of Chemical Structure of Radical Generating Agent with Iodine AbstractionCatalyst on Chain-End Modification Efficiency
3.2. PSt-I Modification
Effect of Type of Iodine Abstraction Catalyst on Chain-End Modification Efficiency
3.3. PBA-I Modification
3.3.1. Effect of Type of Diacyl Peroxide on Chain-End Modification Efficiency
3.3.2. Effect of Dose of Radical Generating Agents on Chain-End Modification Efficiency
3.3.3. Effect of Concentration of Radicals Generated by Decomposition of DiacylPeroxides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fox, T.G.; Flory, P.J. Second-order transition temperatures and related properties of polystyrene influence of molecular weight. J. Appl. Phys. 1950, 21, 581–591. [Google Scholar] [CrossRef]
- Ma, J.; Hashimoto, K.; Koganezawa, T.; Tajima, K. End-on orientation of semiconducting polymers in thin films induced by surface segregation of fluoroalkyl chains. J. Am. Chem. Soc. 2013, 135, 9644–9647. [Google Scholar] [CrossRef] [PubMed]
- Ishiwari, F.; Okabe, G.; Ogiwara, H.; Kajitani, T.; Tokita, M.; Takata, M.; Fukushima, T. Terminal functionalization with a triptycene motif that dramatically changes the structural and physical properties of an amorphous polymer. J. Am. Chem. Soc. 2018, 140, 13497–13502. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, P.; Segui, F.; Shaban, S.; Diab, C.; Okada, Y.; Tanaka, F.; Winnik, F.M. Impact of end-group association and main-chain hydration on the thermosensitive properties of hydrophobically modified telechelic poly(N-isopropylacrylamides) in water. Macromolecules 2006, 39, 341–348. [Google Scholar] [CrossRef]
- Roth, P.J.; Jochum, F.D.; Forst, F.R.; Zentel, R.; Theato, P. Influence of end groups on the stimulus-responsive behavior of poly[oligo(ethylene glycol) methacrylate] in water. Macromolecules 2010, 43, 4638–4645. [Google Scholar] [CrossRef] [Green Version]
- Chauveau, C.; Fouquay, S.; Michaud, G.; Simon, F.; Carpentier, J.; Guillaume, S.M. α,ω-Bis(trialkoxysilyl) telechelic polyolefin/polyether copolymers for adhesive applications using ring-opening insertion metathesis polymerization combined with a chain-transfer agent. ACS Appl. Polym. Mater. 2019, 1, 1540–1546. [Google Scholar] [CrossRef]
- Hagita, K.; Tominaga, T.; Sone, T. Large-scale reverse monte carlo analysis for the morphologies of silica nanoparticles in end-modified rubbers based on ultra-small-angle X-ray scattering data. Polymer 2018, 135, 219–229. [Google Scholar] [CrossRef]
- Goto, A.; Tsujii, Y.; Fukuda, T. Reversible chain transfer catalyzed polymerization (RTCP): A new class of living radical polymerization. Polymer 2008, 49, 5177–5185. [Google Scholar] [CrossRef] [Green Version]
- Georges, M.K.; Veregin, R.P.N.; Kazmaier, P.M.; Hamer, G.K. Narrow molecular weight resins by a free-radical polymerization process. Macromolecules 1993, 26, 2987–2988. [Google Scholar] [CrossRef]
- Yamago, S.; Iida, K.; Yoshida, J. Organotellurium compounds as novel initiators for controlled/living radical polymerizations. Synthesis of functionalized polystyrenes and end-group modifications. J. Am. Chem. Soc. 2002, 124, 2874–2875. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.K.; Frcole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Wang, J.; Matyjaszewski, K. Controlled/“living” radical polymerization. Atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Goto, A.; Suzuki, T.; Ohfuji, H.; Tanishima, M.; Fukuda, T.; Tsujii, Y.; Kaji, H. Reversible complexation mediated living radical polymerization (RCMP) using organic catalysts. Macromolecules 2011, 44, 8709–8715. [Google Scholar] [CrossRef]
- Goto, A.; Zushi, H.; Hirai, N.; Wakada, T.; Tsujii, Y.; Fukuda, T. Living radical polymerizations with germanium, tin, and phosphorus catalysts-reversible chain transfer catalyzed polymerizations (RTCPs). J. Am. Chem. Soc. 2007, 129, 13347–13354. [Google Scholar] [CrossRef]
- Chong, Y.K.; Moad, G.; Rizzardo, E.; Thang, S.H. Thiocarbonylthio end group removal from RAFT-synthesized polymers by radical-induced reduction. Macromolecules 2007, 40, 4446–4455. [Google Scholar] [CrossRef]
- Li, N.; Yang, S.; Huang, Z.; Pan, X. Radical reduction of polymer chain-end functionality by stoichiometric N-heterocyclic carbene boranes. Macromolecules 2021, 54, 6000–6005. [Google Scholar] [CrossRef]
- Yamago, S.; Matsumoto, A. Arylthiols as highly chemoselective and environmentally benign radical reducing agents. J. Org. Chem. 2008, 73, 7300–7304. [Google Scholar] [CrossRef]
- Carmean, R.N.; Figg, C.A.; Scheutz, G.M.; Kubo, T.; Sumerlin, B.S. Catalyst-free photoinduced end-group removal of thiocarbonylthio functionality. ACS Macro Lett. 2017, 6, 185–189. [Google Scholar] [CrossRef]
- Chong, B.; Moad, G.; Rizzardo, E.; Shidmore, M.; Thang, S.H. Thermolysis of RAFT-synthesized poly(methyl methacrylate). Aust. J. Chem. 2006, 59, 755–762. [Google Scholar] [CrossRef]
- Gutekunst, W.R.; Anastasaki, A.; Lunn, D.J.; Truong, N.P.; Whitfield, R.; Jones, G.R.; Treat, N.J.; Abdilla, A.; Barton, B.E.; Clark, P.G.; et al. Practical chain-end reduction of polymers obtained with ATRP. Macromol. Chem. Phys. 2017, 218, 1700107. [Google Scholar] [CrossRef] [Green Version]
- Garamszegi, L.; Donzel, C.; Carrot, G.; Nguyen, T.Q.; Hilborn, J. Synthesis of thiol end-functional polystyrene via atom transfer radical polymerization. React. Funct. Polym. 2003, 55, 179–183. [Google Scholar] [CrossRef]
- Chen, C.; Xiao, L.; Goto, A. Comprehensive study on chain-end transformation of polymer–iodides with amines for synthesizing various chain-end functionalized polymers. Macromolecules 2016, 49, 9425–9440. [Google Scholar] [CrossRef]
- Dietrich, M.; Glassner, M.; Gruendling, T.; Schmid, C.; Falkenhagen, J.; Barner-Kowollik, C. Facile conversion of RAFT polymers into hydroxyl functional polymers: A detailed investigation of variable monomer and RAFT agent combinations. Polym. Chem. 2010, 1, 634–644. [Google Scholar] [CrossRef]
- Heredia, K.L.; Grover, G.N.; Tao, L.; Maynard, H.D. Synthesis of heterotelechelic polymers for conjugation of two different proteins. Macromolecules 2009, 42, 2360–2367. [Google Scholar] [CrossRef] [Green Version]
- Vogt, A.P.; Sumerlin, B.S. An efficient route to macromonomers via ATRP and click chemistry. Macromolecules 2006, 39, 5286–5292. [Google Scholar] [CrossRef]
- Goto, A.; Ohtsuki, A.; Ohfuji, H.; Tanishima, M.; Kaji, H. Reversible generation of a carbon-centered radical from alkyl iodide using organic salts and their application as organic catalysts in living radical polymerization. J. Am. Chem. Soc. 2013, 135, 11131–11139. [Google Scholar] [CrossRef]
- Applications: Free Radical Initiators. Available online: https://www.sigmaaldrich.com/US/en/deepweb/assets/sigmaaldrich/marketing/global/documents/411/888/thermal_initiators.pdf (accessed on 24 April 2023).
- Bulychev, N.A.; Arutunov, I.A.; Zubov, V.P.; Verdonck, B.; Zhang, T.; Goethals, E.J.; Du Prez, F.E. Block copolymers of vinyl ethers as thermo-responsive colloidal stabilizers of organic pigments in aqueous media. Macromol. Chem. Phys. 2004, 205, 2457–2463. [Google Scholar] [CrossRef]
- Schaller, C.; Dirnberger, K.; Schauer, T.; Eisenbach, C.D. Stabilization of carbon black with ionic-hydrophobic polyelectrolytes. Macromol. Symp. 2002, 187, 695–705. [Google Scholar] [CrossRef]
- Chang, J.J.; Pan, H.M.; Goto, A. Synthesis of vinyl iodide chain-end polymers via organocatalyzed chain-end transformation. Chem. Commun. 2021, 57, 1105–1108. [Google Scholar] [CrossRef]
- Kim, K.; Hasneen, A.; Paik, H.; Chang, T. MALDI-TOF MS characterization of polystyrene synthesized by ATRP. Polymer 2013, 54, 6133–6139. [Google Scholar] [CrossRef]
- Ladaviere, C.; Lacroix-Desmazes, P.; Delolme, F. First systematic MALDI/ESI mass spectrometry comparison to characterize polystyrene synthesized by different controlled radical polymerizations. Macromolecules 2009, 42, 70–84. [Google Scholar] [CrossRef]







| Entry | Precursor (eq) | Chain-End Modification Conditions | Molar Mass (×103) | Iodine Chain-End Removal Ratio [%] a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Radical Generating Agent (eq) | Catalyst (eq) | Temp. [°C] | Time [h] | Mn [g/mol] | Mw [g/mol] | PDI [-] | ||||
| 1 b | PMMA-I_1 (1) | AIBN (10) | - | 95 (T1h + 10) | 2 | Before | 7.82 | 8.85 | 1.13 | 0 21 |
| After | 7.93 | 8.96 | 1.13 | |||||||
| 2 b | PMMA-I_1 (1) | LPO (10) | - | 90 (T1h + 10) | 2 | Before | 7.82 | 8.85 | 1.13 | 0 44 |
| After | 7.80 | 8.86 | 1.14 | |||||||
| 3 b | PMMA-I_1 (1) | TCPO (10) | - | 68 (T1h + 10) | 2 | Before | 7.82 | 8.85 | 1.13 | 0 38 |
| After | 7.87 | 9.01 | 1.14 | |||||||
| 4 b | PMMA-I_1 (1) | SAPO (10) | - | 92 (T1h + 5) | 4 | Before | 7.82 | 8.85 | 1.13 | 0 48 |
| After | 7.88 | 9.15 | 1.16 | |||||||
| 5 b | PMMA-I_2 (1) | SAPO (10) | BNI (10) | 92 (T1h + 5) | 4 | Before | 7.29 | 7.98 | 1.09 | 0 72 |
| After | 7.30 | 7.93 | 1.09 | |||||||
| 6 b | PMMA-I_3 (1) | ACVA (10) | BNI (10) | 94 (T1h + 5) | 4 | Before | 7.60 | 8.24 | 1.08 | 0 33 |
| After | 7.77 | 8.62 | 1.11 | |||||||
| Measured Polymer | Observed Polymer | Structure | N | m/z | Chain-End Modification ratio a [%] | |
|---|---|---|---|---|---|---|
| Theoretical (with Na+) | Experimental (with Na+) | |||||
| PMMA-I_2 | P1 |  | 69 | 7091.4 | 7091.6 | 63 |
| P2 |  | 69 | 7077.4 | 7078.6 | 37 | |
| Entry 5 | P3 |  | 68 | 7109.4 | 7111.5 | 30 |
| P4 |  | 69 | 7093.4 | 7093.5 | 10 | |
| P5(P2) |  | 69 | 7077.4 | 7078.5 | 60 | |
| Entry | Precursor (eq) | Chain-End Modification Condition | Molar Mass (×103) | Iodine Chain-End Removal Ratio [%] a | Chain-End Modification Ratio [%] b | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Radical Generating Agent (eq) | Iodine Abstraction Catalyst (eq) | Temp. [°C] | Time [h] | Mn [g/mol] | Mw [g/mol] | PDI [-] | ||||
| 7 | PSt-I (1) | - | - | - | - | 3.51 | 4.36 | 1.24 | - | - |
| 8 c | PSt-I’ d (1) | LPO (10) | BNI (10) | 80 (T1h + 0) | 4 | 4.01 | 4.60 | 1.15 | 100 | 27 |
| 8′ c | PSt-I (1) | LPO (2) | BNI (5) | 80 (T1h + 0) | 4 | 3.98 | 4.68 | 1.18 | 100 | 29 |
| 9 c | PSt-I (1) | LPO (2) | BNBr (5) | 80 (T1h + 0) | 4 | 3.93 | 4.69 | 1.19 | 100 | 33 |
| 10 c | PSt-I (1) | LPO (2) | BSI (5) | 80 (T1h + 0) | 4 | 3.91 | 4.58 | 1.17 | 34 | 0 |
| Entry | Precursor (eq) | Chain-End Modification Condition | Molar Mass (× 103) | Iodine Chain-End Removal Ratio [%] a | Chain-End Modification Ratio [%] b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Radical Generating Agent | Iodine Abstraction Catalyst (eq) | Temp. [°C] | Time [h] | Mn [g/mol] | Mw [g/mol] | PDI [-] | |||||
| Abbrev. (eq) | Moiety | ||||||||||
| 11 | PBA-I (1) | - | - | - | - | - | 6.34 | 7.40 | 1.17 | - | - |
| 12 c | PBA-I (1) | LPO (10) |  | BNI (10) | 110 (T1h + 30) | 1 | 6.24 | 7.26 | 1.16 | 100 | 100 |
| 13 c | PBA-I (1) | BPO (10) |  | BNI (10) | 110 (T1h + 18) | 1 | 6.59 | 7.72 | 1.17 | 100 | 85 |
| 14 d | PBA-I (1) | SAPO (10) |  | BNI (10) | 110 (T1h + 23) | 1 | 6.34 | 7.33 | 1.16 | 83 | 25 |
| Entry | Precursor (eq) | Chain-End Modification Condition | Molar Mass (×103) | Iodine Chain-end Removal ratio [%] a | Chain-end Modification Ratio [%] b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Radical Generating Agent | Iodine Abstraction Catalyst (eq) | Temp. [°C] | Time [h] | Mn [g/mol] | Mw [g/mol] | PDI [-] | |||||
| Abbrev. (eq) | Moiety | ||||||||||
| 12 c | PBA-I (1) | LPO (10) |  | BNI (10) | 110 (T1h + 30) | 1 | 6.24 | 7.26 | 1.16 | 100 | 100 |
| 15 c | PBA-I (1) | LPO (5) | BNI (10) | 110 (T1h + 30) | 1 | 6.52 | 7.56 | 1.16 | 100 | 100 | |
| 16 c | PBA-I (1) | LPO (2) | BNI (10) | 110 (T1h + 30) | 1 | 6.62 | 7.59 | 1.15 | 100 | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohtani, K.; Shimizu, K.; Takahashi, T.; Takamura, M. Novel Chain-End Modification of Polymer Iodides via Reversible Complexation-Mediated Polymerization with Functionalized Radical Generation Agents. Polymers 2023, 15, 2667. https://doi.org/10.3390/polym15122667
Ohtani K, Shimizu K, Takahashi T, Takamura M. Novel Chain-End Modification of Polymer Iodides via Reversible Complexation-Mediated Polymerization with Functionalized Radical Generation Agents. Polymers. 2023; 15(12):2667. https://doi.org/10.3390/polym15122667
Chicago/Turabian StyleOhtani, Kazuya, Kanta Shimizu, Tatsuhiro Takahashi, and Masumi Takamura. 2023. "Novel Chain-End Modification of Polymer Iodides via Reversible Complexation-Mediated Polymerization with Functionalized Radical Generation Agents" Polymers 15, no. 12: 2667. https://doi.org/10.3390/polym15122667
APA StyleOhtani, K., Shimizu, K., Takahashi, T., & Takamura, M. (2023). Novel Chain-End Modification of Polymer Iodides via Reversible Complexation-Mediated Polymerization with Functionalized Radical Generation Agents. Polymers, 15(12), 2667. https://doi.org/10.3390/polym15122667