A Review on Reinforcements and Additives in Starch-Based Composites for Food Packaging
Abstract
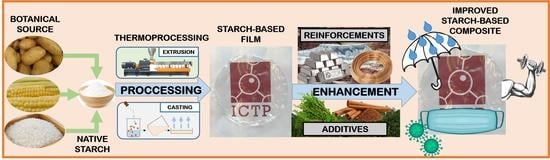
:1. Introduction
2. Starch
2.1. Starch Sources and Extraction
| Plant Source | Size (µm) | Shape |
|---|---|---|
| Maize | 2–30 | Round and polyhedral |
| Waxy maize | 2–30 | Round and polyhedral |
| Wheat | 10 and 10–30 | Discs |
| Waxy wheat | >10 | Spherical and lenticular |
| Rice | <20 and 2–8 | Polygonal and angular |
| Tapioca | 5–45 | Spherical/lenticular |
| Cassava | 30 | Kettle-drum truncated |
| Potato | <110 | Oval and irregular |
| Sweet Potato | 1–100 | Oval, spherical, round |
| Pea | 2–40 | Oval, spherical, round |
| Lotus | 14–31 | Oblong |

2.2. Thermoplastic Starch, TPS
2.3. Preparation of Starch-Based Films
2.3.1. Solvent Casting
2.3.2. Tape Casting
2.3.3. Extrusion
2.3.4. Film Blowing
3. Nanoparticles
3.1. Inorganic Nanofillers
3.1.1. Nanoclays
Montmorillonite (MMT)
Other Nanoclays
3.1.2. Metal Nanoparticles
Silver Nanoparticles (Ag NPs)
- (1)
- Ag2+ interacts with sulfhydryl groups in the cell wall to generate insoluble compounds that are parts of several enzymes involved in the production of transmembrane energy and the transport of electrolytes.
- (2)
- Ag2+ inhibits the cytochrome oxidase and NADH-succinate-dehydrogenase regions of the bacterial respiratory chain.
- (3)
Zinc Oxide (ZnO) NP
Titanium Dioxide (TiO2) NP
Copper Oxide (CuO)
Other Metallic NPs
3.2. Organic Nanofillers
3.2.1. Starch Nanomaterials
3.2.2. Cellulose Nanomaterials


Cellulose Nanocrystals (CNC)
Cellulose Nanofibers (CNF)
Bacterial Cellulose (BC)
3.2.3. Chitin/Chitosan Nanoparticles
4. Additives
4.1. Plasticizers
4.2. Essential Oils (EOs)
- Terpenes: With more than 40,000 distinct chemical structures, terpenes are one of the principal constituents of essential oils and one of the most abundant and diverse categories of plant natural products [266]. There are at least two possible ways to biosynthesize terpenes: from mevalonic acid or from metileritritol phosphate [267]. However, all terpenes are derivates of the isoprene structure (Figure 15).

- Polyphenols: These molecules can be divided into different classes depending on their chemical structure. Polyphenols are found in nature as a product of the secondary metabolism of most plants [269]. Two main classes of phenolic acids can be distinguished depending on their structure. Both of them have benzene as a basic bond to a carboxylic group (benzoic acids) or to a propionic group (cinnamic acids), and both structures can be found with different hydroxylation levels [270]. In addition, some examples of molecules inside this group that are not phenolic acids are: coumarins, lignans, chalcones, flavonoids, lignins, and stilbenes [271].
4.2.1. Rosemary Essential Oil (REO)
4.2.2. Cinnamon Essential Oil (CEO)
4.2.3. Thyme Essential Oil (TEO)
4.3. Organic Molecules
4.3.1. Carvacrol
4.3.2. Eugenol
4.3.3. Cinnamic Acids and Derivates
4.3.4. Organic Molecules in Smart Packaging

| Matrix | Indicator | Botanical Source | Preparation Method | Food Tested | Color Change | Ref. |
|---|---|---|---|---|---|---|
| cassava starch | anthocyanin | grape skin | extrusion | meat | black to purple | [330] |
| starch + PVA | anthocyanin | roselle calyces | solvent casting | fish | pink to blue to yellow | [331] |
| agar/potato starch | anthocyanin | purple sweet potato | - | meat | red to green | [332] |
| starch + gelatin | anthocyanin | purple sweet potato | solvent casting | mushrooms | green to gray to yellow | [333] |
| native/hydrolyzed cassava starch | anthocyanin | yerba mate | extrusion | - | white to yellow/brown | [334] |
| sago starch | anthocyanin | red cabbage | solvent casting | - | pink to green to yellow | [320] |
| starch + gelatin | anthocyanin + [KI/DPC/Fe(III)] | red cabbage | solvent casting | milk | pink to blue to yellow/green | [328] |
| cassava starch + PVA | encapsulated anthocyanin | black fruit wolfberry | solvent casting | fish | pink to blue to yellow/green | [329] |
| cassava starch + PVA | betanin | beetroot red | solvent casting | - | red brown to brownish yellow | [335] |
| potato starch | betacyanin | paperflower | solvent casting | fish | pink to yellow | [336] |
| corn starch + PVA | curcumin | - | microwave-ultrasound reaction | fish | yellow to red | [337] |
| TPS + PE | curcumin | - | extrusion | meat | light yellow to light brown | [338] |
| corn starch | shikonin | zicao roots | solvent casting | shrimps | red to blue | [339] |
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Filho, W.L.; Salvia, A.L.; Bonoli, A.; Saari, U.A.; Voronova, V.; Klõga, M.; Kumbhar, S.S.; Olszewski, K.; De Quevedo, D.M.; Barbir, J. An Assessment of Attitudes towards Plastics and Bioplastics in Europe. Sci. Total Environ. 2021, 755, 142732. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe. Plastics-the Facts 2022 OCTOBER. 2022. Available online: https://plasticseurope.org/wp-content/uploads/2022/10/PE-PLASTICS-THE-FACTS_V7-Tue_19-10-1.pdf (accessed on 1 May 2023).
- Beaumont, N.J.; Aanesen, M.; Austen, M.C.; Börger, T.; Clark, J.R.; Cole, M.; Hooper, T.; Lindeque, P.K.; Pascoe, C.; Wyles, K.J. Global Ecological, Social and Economic Impacts of Marine Plastic. Mar. Pollut. Bull. 2019, 142, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic Waste Inputs from Land into the Ocean. Science 1979 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Borrelle, S.B.; Ringma, J.; Law, K.L.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Murphy, E.; Jambeck, J.; Leonard, G.H.; Hilleary, M.A.; et al. Predicted Growth in Plastic Waste Exceeds Efforts to Mitigate Plastic Pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef]
- Phelan, A.A.; Meissner, K.; Humphrey, J.; Ross, H. Plastic Pollution and Packaging: Corporate Commitments and Actions from the Food and Beverage Sector. J. Clean. Prod. 2022, 331, 129827. [Google Scholar] [CrossRef]
- Kan, M.; Miller, S.A. Environmental Impacts of Plastic Packaging of Food Products. Resour. Conserv. Recycl. 2022, 180, 106156. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J. The Effects of Environmental Conditions on the Structural Features and Physico-Chemical Properties of Starches. Starch-Staerke 2001, 53, 513–519. [Google Scholar] [CrossRef]
- Magallanes-Cruz, P.A.; Duque-Buitrago, L.F.; del Rocío Martínez-Ruiz, N. Native and Modified Starches from Underutilized Seeds: Characteristics, Functional Properties and Potential Applications. Food Res. Int. 2023, 169, 112875. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, P.; Li, Y.; Li, J.; Li, X.; Yang, J.; Ji, M.; Li, F.; Zhang, C. Recent Advances and Future Challenges of the Starch-Based Bio-Composites for Engineering Applications. Carbohydr. Polym. 2023, 307, 120627. [Google Scholar] [CrossRef]
- Carlstedt, J.; Wojtasz, J.; Fyhr, P.; Kocherbitov, V. Understanding Starch Gelatinization: The Phase Diagram Approach. Carbohydr. Polym. 2015, 129, 62–69. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Y.; Wang, J.; Wang, S.; Copeland, L. Molecular Disassembly of Rice and Lotus Starches during Thermal Processing and Its Effect on Starch Digestibility. Food Funct. 2016, 7, 1188–1195. [Google Scholar] [CrossRef]
- Majeed, T.; Dar, A.H.; Pandey, V.K.; Dash, K.K.; Srivastava, S.; Shams, R.; Jeevarathinam, G.; Singh, P.; Echegaray, N.; Pandiselvam, R. Role of Additives in Starch-Based Edible Films and Coating: A Review with Current Knowledge. Prog. Org. Coat. 2023, 181, 107597. [Google Scholar] [CrossRef]
- Karimi Sani, I.; Masoudpour-Behabadi, M.; Alizadeh Sani, M.; Motalebinejad, H.; Juma, A.S.M.; Asdagh, A.; Eghbaljoo, H.; Khodaei, S.M.; Rhim, J.W.; Mohammadi, F. Value-Added Utilization of Fruit and Vegetable Processing by-Products for the Manufacture of Biodegradable Food Packaging Films. Food Chem. 2023, 405, 134964. [Google Scholar] [CrossRef]
- Farajpour, R.; Emam Djomeh, Z.; Moeini, S.; Tavahkolipour, H.; Safayan, S. Structural and Physico-Mechanical Properties of Potato Starch-Olive Oil Edible Films Reinforced with Zein Nanoparticles. Int. J. Biol. Macromol. 2020, 149, 941–950. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-Based Films: Major Factors Affecting Their Properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef]
- Arora, A.; Padua, G.W. Review: Nanocomposites in Food Packaging. J. Food Sci. 2010, 75, 43–49. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Oleyaei, S.A.; Almasi, H. Nanostructured Materials Utilized in Biopolymer-Based Plastics for Food Packaging Applications. Crit. Rev. Food Sci. Nutr. 2015, 55, 1699–1723. [Google Scholar] [CrossRef]
- García, N.L.; Famá, L.; D’Accorso, N.B.; Goyanes, S. Biodegradable Starch Nanocomposites. Adv. Struct. Mater. 2015, 75, 17–77. [Google Scholar] [CrossRef]
- Niranjana Prabhu, T.; Prashantha, K. A Review on Present Status and Future Challenges of Starch Based Polymer Films and Their Composites in Food Packaging Applications. Polym. Compos. 2018, 39, 2499–2522. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, P.; Chen, J.; Yan, Y.; Wu, Z. Recent Advances and Applications in Starch for Intelligent Active Food Packaging: A Review. Foods 2022, 11, 2879. [Google Scholar] [CrossRef]
- Jane, J.; Chen, Y.Y.; Lee, L.F.; McPherson, A.E.; Wong, K.S.; Radosavljevic, M.; Kasemsuwan, T. Effects of Amylopectin Branch Chain Length and Amylose Content on the Gelatinization and Pasting Properties of Starch. Cereal Chem. 1999, 76, 629–637. [Google Scholar] [CrossRef]
- Willfahrt, A.; Steiner, E.; Hötzel, J.; Crispin, X. Printable Acid-Modified Corn Starch as Non-Toxic, Disposable Hydrogel-Polymer Electrolyte in Supercapacitors. Appl. Phys. A Mater. Sci. Process 2019, 125, 474. [Google Scholar] [CrossRef]
- Singh Sandhu, K.; Singh, N. Relationships between Selected Properties of Starches from Different Corn Lines. Int. J. Food Prop. 2005, 8, 481–491. [Google Scholar] [CrossRef]
- Singh, G.D.; Bawa, A.S.; Singh, S.; Saxena, D.C. Physicochemical, Pasting, Thermal and Morphological Characteristics of Indian Water Chestnut (Trapa natans) Starch. Starch-Staerke 2009, 61, 35–42. [Google Scholar] [CrossRef]
- Mohammed, A.A.B.A.; Hasan, Z.; Omran, A.A.B.; Kumar, V.V.; Elfaghi, A.M.; Ilyas, R.A.; Sapuan, S.M. Corn: Its Structure, Polymer, Fiber, Composite, Properties, and Applications. Polymers 2022, 14, 4396. [Google Scholar] [CrossRef]
- Ibrahim, M.I.J.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M. Extraction, chemical composition, and characterization of potential lignocellulosic biomasses and polymers from corn plant parts. BioResources 2019, 14, 6485–6500. [Google Scholar] [CrossRef]
- Marichelvam, M.K.; Jawaid, M.; Asim, M. Corn and Rice Starch-Based Bio-Plastics as Alternative Packaging Materials. Fibers 2019, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Yu, L.; Xie, F.; Chen, L. Gelatinization of Cornstarch with Different Amylose/Amylopectin Content. Carbohydr. Polym. 2006, 65, 357–363. [Google Scholar] [CrossRef]
- Fredrikssona, H.; Silveriob, J.; Andemon’, R.; Eliassonb, A.-C.; Amanta, P. The Influence of Amylose and Amylopectin Characteristics on Gelatinization and Retrogradation Properties of Different Starches. Carbohydr. Polym. 1998, 35, 119–134. [Google Scholar] [CrossRef]
- Dhital, S.; Shrestha, A.K.; Hasjim, J.; Gidley, M.J. Physicochemical and Structural Properties of Maize and Potato Starches as a Function of Granule Size. J. Agric. Food Chem. 2011, 59, 10151–10161. [Google Scholar] [CrossRef]
- Simsek, S.; Ovando-Martinez, M.; Marefati, A.; Sj, M.; Rayner, M. Chemical Composition, Digestibility and Emulsification Properties of Octenyl Succinic Esters of Various Starches. Food Res. Int. 2015, 75, 41–49. [Google Scholar] [CrossRef]
- Waterschoot, J.; Gomand, S.V.; Fierens, E.; Delcour, J.A. Production, Structure, Physicochemical and Functional Properties of Maize, Cassava, Wheat, Potato and Rice Starches. Starch-Staerke 2015, 67, 14–29. [Google Scholar] [CrossRef]
- Vandeputte, G.E.; Vermeylen, R.; Geeroms, J.; Delcour, J.A. Rice Starches. I. Structural Aspects Provide Insight into Crystallinity Characteristics and Gelatinisation Behaviour of Granular Starch. J. Cereal Sci. 2003, 38, 43–52. [Google Scholar] [CrossRef]
- Juliano, B.O.; Villareal, R.M.; Perez, C.M.; Villareal, C.P.; Baños, L.; Takeda, V.; Hizukuri, S. Varietal Differences in Properties Among High Amylose Rice Starches. Starch-Stärke 1987, 39, 390–393. [Google Scholar] [CrossRef]
- Singh, N.; Kaur, L.; Sandhu, K.S.; Kaur, J.; Nishinari, K. Relationships between Physicochemical, Morphological, Thermal, Rheological Properties of Rice Starches. Food Hydrocoll. 2006, 20, 532–542. [Google Scholar] [CrossRef]
- Singh, N.; Singh, J.; Kaur, L.; Sodhi, N.S.; Gill, S. Morphological, Thermal and Rheological Properties of Starches from Different Botanical Sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
- Kumari, S.; Yadav, B.S.; Yadav, R.B. Synthesis and Modification Approaches for Starch Nanoparticles for Their Emerging Food Industrial Applications: A Review. Food Res. Int. 2020, 128, 108765. [Google Scholar] [CrossRef]
- Domene-López, D.; García-Quesada, J.C.; Martin-Gullon, I.; Montalbán, M.G. Influence of Starch Composition and Molecular Weight on Physicochemical Properties of Biodegradable Films. Polymers 2019, 11, 1084. [Google Scholar] [CrossRef] [Green Version]
- García, N.L.; Ribba, L.; Dufresne, A.; Aranguren, M.; Goyanes, S. Effect of Glycerol on the Morphology of Nanocomposites Made from Thermoplastic Starch and Starch Nanocrystals. Carbohydr. Polym. 2011, 84, 203–210. [Google Scholar] [CrossRef]
- Khan, B.; Bilal Khan Niazi, M.; Samin, G.; Jahan, Z. Thermoplastic Starch: A Possible Biodegradable Food Packaging Material—A Review. J. Food Process Eng. 2017, 40, e12447. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Ferreira, D.C.; Louzada, L.B.; Dos Santos, F.; Corrêa, A.C.; Moreira, F.K.V.; Mattoso, L.H. Starch-Based Edible Films and Coatings: An Eco-Friendly Alternative for Food Packaging; Academic Press: Cambridge, MA, USA, 2018; ISBN 9780128094402. [Google Scholar]
- Agarwal, S. Major Factors Affecting the Characteristics of Starch Based Biopolymer Films. Eur. Polym. J. 2021, 160, 110788. [Google Scholar] [CrossRef]
- Li, C. Recent Progress in Understanding Starch Gelatinization—An Important Property Determining Food Quality. Carbohydr. Polym. 2022, 293, 119735. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hu, Y. A Kinetics-Based Decomposition Approach to Reveal the Nature of Starch Asymmetric Gelatinization Thermograms at Non-Isothermal Conditions. Food Chem. 2021, 344, 128697. [Google Scholar] [CrossRef] [PubMed]
- Varavinit, S.; Shobsngob, S.; Varanyanond, W.; Chinachoti, P.; Naivikul, O. Effect of Amylose Content on Gelatinization, Retrogradation and Pasting Properties of Flours from Different Cultivars of Thai Rice. Starch-Staerke 2003, 55, 410–415. [Google Scholar] [CrossRef]
- Mathew, A.P.; Dufresne, A. Morphological Investigation of Nanocomposites from Sorbitol Plasticized Starch and Tunicin Whiskers. Biomacromolecules 2002, 3, 609–617. [Google Scholar] [CrossRef]
- Ma, X.; Yu, J. Formamide as the Plasticizer for Thermoplastic Starch. J. Appl. Polym. Sci. 2004, 93, 1769–1773. [Google Scholar] [CrossRef]
- Nawab, A.; Alam, F.; Haq, M.A.; Lutfi, Z.; Hasnain, A. Mango Kernel Starch-Gum Composite Films: Physical, Mechanical and Barrier Properties. Int. J. Biol. Macromol. 2017, 98, 869–876. [Google Scholar] [CrossRef]
- Van Soest, J.J.G.; Vliegenthart, J.F.G. Crystallinity in Starch Plastics: Consequences for Material Properties. Trends Biotechnol. 1997, 15, 208–213. [Google Scholar] [CrossRef]
- Chen, Y.L.; Shull, K.R. Controlling the Properties of Thermoplastic Starch Films with Hydrogen Bonding Plasticizers. Carbohydr. Polym. Technol. Appl. 2023, 5, 100291. [Google Scholar] [CrossRef]
- Luchese, C.L.; Spada, J.C.; Tessaro, I.C. Starch Content Affects Physicochemical Properties of Corn and Cassava Starch-Based Films. Ind. Crops Prod. 2017, 109, 619–626. [Google Scholar] [CrossRef]
- Averous, L.; Boquillon, N. Biocomposites Based on Plasticized Starch: Thermal and Mechanical Behaviours. Carbohydr. Polym. 2004, 56, 111–122. [Google Scholar] [CrossRef]
- Angellier, H.; Molina-Boisseau, S.; Dole, P.; Dufresne, A. Thermoplastic Starch—Waxy Maize Starch Nanocrystals Nanocomposites. Biomacromolecules 2006, 7, 531–539. [Google Scholar] [CrossRef]
- Ceseracciu, L.; Heredia-Guerrero, J.A.; Dante, S.; Athanassiou, A.; Bayer, I.S. Robust and Biodegradable Elastomers Based on Corn Starch and Polydimethylsiloxane (PDMS). ACS Appl. Mater. Interfaces 2015, 7, 3742–3753. [Google Scholar] [CrossRef]
- Müller, C.M.O.; Yamashita, F.; Laurindo, J.B. Evaluation of the Effects of Glycerol and Sorbitol Concentration and Water Activity on the Water Barrier Properties of Cassava Starch Films through a Solubility Approach. Carbohydr. Polym. 2008, 72, 82–87. [Google Scholar] [CrossRef]
- Li, J.; Luo, X. Strach-Based Blends. In Starch-Based Blends, Composites and Nanocomposites; Royal Society of Chemistry: London, UK, 2016; pp. 263–325. [Google Scholar]
- Siemann, U. Solvent Cast Technology—A Versatile Tool for Thin Film Production. Prog. Colloid. Polym. Sci. 2005, 130, 1–14. [Google Scholar] [CrossRef]
- Liu, H.; Xie, F.; Yu, L.; Chen, L.; Li, L. Thermal Processing of Starch-Based Polymers. Prog. Polym. Sci. 2009, 34, 1348–1368. [Google Scholar] [CrossRef]
- Sessini, V.; Arrieta, M.P.; Kenny, J.M.; Peponi, L. Processing of Edible Films Based on Nanoreinforced Gelatinized Starch. Polym. Degrad. Stab. 2016, 132, 157–168. [Google Scholar] [CrossRef]
- Müller, C.M.O.; Laurindo, J.B.; Yamashita, F. Effect of Nanoclay Incorporation Method on Mechanical and Water Vapor Barrier Properties of Starch-Based Films. Ind. Crops Prod. 2011, 33, 605–610. [Google Scholar] [CrossRef]
- Richard, E.; Mistler, E.R.T. Tape Casting: Theory and Practice; Wiley: Hoboken, NJ, USA, 2000; ISBN 978-1-574-98029-5. [Google Scholar]
- do Val Siqueira, L.; Arias, C.I.L.F.; Maniglia, B.C.; Tadini, C.C. Starch-Based Biodegradable Plastics: Methods of Production, Challenges and Future Perspectives. Curr. Opin. Food Sci. 2021, 38, 122–130. [Google Scholar] [CrossRef]
- Oliveira de Moraes, J.; Scheibe, A.S.; Augusto, B.; Carciofi, M.; Laurindo, J.B. Conductive Drying of Starch-Fiber Films Prepared by Tape Casting: Drying Rates and Film Properties. LWT—Food Sci. Technol. 2015, 64, 356–366. [Google Scholar] [CrossRef]
- De Moraes, J.O.; Scheibe, A.S.; Sereno, A.; Laurindo, J.B. Scale-up of the Production of Cassava Starch Based Films Using Tape-Casting. J. Food Eng. 2013, 119, 800–808. [Google Scholar] [CrossRef] [Green Version]
- Scheibe, A.S.; De Moraes, J.O.; Laurindo, J.B. Production and Characterization of Bags from Biocomposite Films of Starch-Vegetal Fibers Prepared by Tape Casting. J. Food Process Eng. 2014, 37, 482–492. [Google Scholar] [CrossRef]
- Nishihora, R.K.; Rachadel, P.L.; Quadri, M.G.N.; Hotza, D. Manufacturing Porous Ceramic Materials by Tape Casting—A Review. J. Eur. Ceram. Soc. 2018, 38, 988–1001. [Google Scholar] [CrossRef]
- Von Borries-Medrano, E.; Jaime-Fonseca, M.R.; Aguilar-Méndez, M.A. Starch-Guar Gum Extrudates: Microstructure, Physicochemical Properties and in-Vitro Digestion. Food Chem. 2016, 194, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Harnkarnsujarit, N.; Wongphan, P.; Chatkitanan, T.; Laorenza, Y.; Srisa, A. Bioplastic for Sustainable Food Packaging; Elsevier Inc.: Amsterdam, The Netherlands, 2021; ISBN 9780128227145. [Google Scholar]
- González-Seligra, P.; Guz, L.; Ochoa-Yepes, O.; Goyanes, S.; Famá, L. Influence of Extrusion Process Conditions on Starch Film Morphology. LWT 2017, 84, 520–528. [Google Scholar] [CrossRef]
- Li, M.; Liu, P.; Zou, W.; Yu, L.; Xie, F.; Pu, H.; Liu, H.; Chen, L. Extrusion Processing and Characterization of Edible Starch Films with Different Amylose Contents. J. Food Eng. 2011, 106, 95–101. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z.; Liu, J.; Dai, B.; Hu, S.; Hong, R.; Xie, H.; Li, Z.; Chen, Y.; Zeng, G. Preparation, Reinforcement and Properties of Thermoplastic Starch Film by Film Blowing. Food Hydrocoll. 2020, 108, 106006. [Google Scholar] [CrossRef]
- Rejak, A.; Moscicki, L. TPS Film—Blowing. In Thermoplastic Starch. A Green Material for Various Industries; Janssen, L.P.B.M., Moscicki, L., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2009; pp. 173–184. ISBN 978-3-527-32528-3. [Google Scholar]
- Dang, K.M.; Yoksan, R. Thermoplastic Starch Blown Films with Improved Mechanical and Barrier Properties. Int. J. Biol. Macromol. 2021, 188, 290–299. [Google Scholar] [CrossRef]
- Palai, B.; Biswal, M.; Mohanty, S.; Nayak, S.K. In Situ Reactive Compatibilization of Polylactic Acid (PLA) and Thermoplastic Starch (TPS) Blends; Synthesis and Evaluation of Extrusion Blown Films Thereof. Ind. Crops Prod. 2019, 141, 111748. [Google Scholar] [CrossRef]
- Matzinos, P.; Tserki, V.; Kontoyiannis, A.; Panayiotou, C. Processing and Characterization of Starch/Polycaprolactone Products. Polym. Degrad. Stab. 2002, 77, 17–24. [Google Scholar] [CrossRef]
- de Campos, S.S.; de Oliveira, A.; Moreira, T.F.M.; da Silva, T.B.V.; da Silva, M.V.; Pinto, J.A.; Bilck, A.P.; Gonçalves, O.H.; Fernandes, I.P.; Barreiro, M.F.; et al. TPCS/PBAT Blown Extruded Films Added with Curcumin as a Technological Approach for Active Packaging Materials. Food Packag. Shelf Life 2019, 22, 100424. [Google Scholar] [CrossRef] [Green Version]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Blown Film Extrusion of PBAT/TPS/ZnO Nanocomposites for Shelf-Life Extension of Meat Packaging. Colloids Surf. B Biointerfaces 2022, 214, 112472. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Grossmann, M.V.E.; Yamashita, F.; Pined, E.A.G. Antimicrobial, Mechanical, and Barrier Properties of Cassava Starch-Chitosan Films Incorporated with Oregano Essential Oil. J. Agric. Food Chem. 2009, 57, 7499–7504. [Google Scholar] [CrossRef]
- Cho, J.; Joshi, M.S.; Sun, C.T. Effect of Inclusion Size on Mechanical Properties of Polymeric Composites with Micro and Nano Particles. Compos. Sci. Technol. 2006, 66, 1941–1952. [Google Scholar] [CrossRef]
- Zare, Y. The Roles of Nanoparticles Accumulation and Interphase Properties in Properties of Polymer Particulate Nanocomposites by a Multi-Step Methodology. Compos. Part A Appl. Sci. Manuf. 2016, 91, 127–132. [Google Scholar] [CrossRef]
- Nguyen, V.S.; Rouxel, D.; Vincent, B. Dispersion of Nanoparticles: From Organic Solvents to Polymer Solutions. Ultrason. Sonochem. 2014, 21, 149–153. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, H.; Zhang, Z. Homogeneous Nanoparticle Dispersion Prepared with Impurity-Free Dispersant by the Ball Mill Technique. Particuology 2013, 11, 441–447. [Google Scholar] [CrossRef]
- Wolf, C.; Angellier-Coussy, H.; Gontard, N.; Doghieri, F.; Guillard, V. How the Shape of Fillers Affects the Barrier Properties of Polymer/Non-Porous Particles Nanocomposites: A Review. J. Memb. Sci. 2018, 556, 393–418. [Google Scholar] [CrossRef]
- Lengowski, E.C.; Bonfatti Júnior, E.A.; de Muñiz, G.I.B.; Satyanarayana, K.G. Barrier Properties of Nanoparticle-Based Polymer Composites. In Nanoparticle-Based Polymer Composites; Woodhead Publishing: Sawston, UK, 2022; pp. 219–241. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu. Bionanocomposites: An Overview; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; ISBN 9780128212806. [Google Scholar]
- Amenta, V.; Aschberger, K.; Arena, M.; Bouwmeester, H.; Botelho, F.; Brandhoff, P.; Gottardo, S.; Marvin, H.J.P.; Mech, A.; Quiros, L.; et al. Regulatory Aspects of Nanotechnology in the Agri/Feed/Food Sector in EU and Non-EU Countries. Regul. Toxicol. Pharmacol. 2015, 73, 463–476. [Google Scholar] [CrossRef]
- Food and Drug Administration (US). 21 CFR Part 11 Title 21—Food and Drugs Chapter I—Food and Drug Administration, Department of Health and Human Services Subchapter A—General; Food and Drug Administration: Silver Spring, MD, USA, 2023; Volume 575, pp. 1–2. [Google Scholar]
- European Commission Commission Regulation (EU). 2022/63 of 14 January 2022 Amending Annexes II and III to Regulation (EC) No 1333/2008 of European Parliament and of the Council as Regards the Food Additive Titanium Dioxide (E171). Off. J. Eur. Union 2022, 11, 1–5. [Google Scholar]
- European Parliament and Council. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers; European Parliament and Council: Brussels, Belgium, 2011; Volume L 304. [Google Scholar]
- Simoneau, C. Guidelines on Testing Conditions for Articles in Contact with Foodstuffs; European Communities: Brussels, Belgium, 2009. [Google Scholar]
- Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mengelers, M.; et al. Safety Assessment of the Substance Silver Nanoparticles for Use in Food Contact Materials. EFSA J. 2021, 19, e06790. [Google Scholar] [CrossRef] [PubMed]
- Peighambardoust, S.J.; Peighambardoust, S.H.; Mohammadzadeh Pournasir, N.; Pakdel, P. Properties of Active Starch-Based Films Incorporating a Combination of Ag, ZnO and CuO Nanoparticles for Potential Use in Food Packaging Applications. Food Packag. Shelf Life 2019, 22, 100420. [Google Scholar] [CrossRef]
- das Neves, M.D.S.; Scandorieiro, S.; Pereira, G.N.; Ribeiro, J.M.; Seabra, A.B.; Dias, A.P.; Yamashita, F.; Martinez, C.B.D.R.; Kobayashi, R.K.T.; Nakazato, G. Antibacterial Activity of Biodegradable Films Incorporated with Biologically-Synthesized Silver Nanoparticles and the Evaluation of Their Migration to Chicken Meat. Antibiotics 2023, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Nath, D.; Santhosh, R.; Pal, K.; Sarkar, P. Nanoclay-Based Active Food Packaging Systems: A Review. Food Packag. Shelf Life 2022, 31, 100803. [Google Scholar] [CrossRef]
- Choudalakis, G.; Gotsis, A.D. Permeability of Polymer/Clay Nanocomposites: A Review. Eur. Polym. J. 2009, 45, 967–984. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Zhou, W. Safety Assessment of Nanocomposite for Food Packaging Application. Trends Food Sci. Technol. 2015, 45, 187–199. [Google Scholar] [CrossRef]
- Silvestre, C.; Duraccio, D.; Cimmino, S. Food Packaging Based on Polymer Nanomaterials. Prog. Polym. Sci. 2011, 36, 1766–1782. [Google Scholar] [CrossRef]
- Rafiee, R.; Shahzadi, R. Mechanical Properties of Nanoclay and Nanoclay Reinforced Polymers: A Review. Polym. Compos. 2019, 40, 431–445. [Google Scholar] [CrossRef]
- Tang, X.; Alavi, S.; Herald, T.J. Barrier and Mechanical Properties of Starch-Clay Nanocomposite Films. Cereal Chem. 2008, 85, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Girdthep, S.; Worajittiphon, P.; Molloy, R.; Leejarkpai, T.; Punyodom, W. Formulation and Characterization of Compatibilized Poly(Lactic Acid)-Based Blends and Their Nanocomposites with Silver-Loaded Kaolinite. Polym. Int. 2015, 64, 203–211. [Google Scholar] [CrossRef]
- Osman, A.F.; Siah, L.; Alrashdi, A.A.; Ul-Hamid, A.; Ibrahim, I. Improving the Tensile and Tear Properties of Thermoplastic Starch/Dolomite Biocomposite Film through Sonication Process. Polymers 2021, 13, 274. [Google Scholar] [CrossRef]
- Gao, W.; Dong, H.; Hou, H.; Zhang, H. Effects of Clays with Various Hydrophilicities on Properties of Starch–Clay Nanocomposites by Film Blowing. Carbohydr. Polym. 2012, 88, 321–328. [Google Scholar] [CrossRef]
- Ruamcharoen, J.; Munlee, R.; Ruamcharoen, P. Eco-Friendly Bio-Based Composites of Cassava Starch and Natural Rubber Compatibilized with Nanoclays. Polym. Compos. 2023, 44, 1071–1082. [Google Scholar] [CrossRef]
- Cheviron, P.; Gouanvé, F.; Espuche, E. Preparation, Characterization and Barrier Properties of Silver/Montmorillonite/Starch Nanocomposite Films. J. Memb. Sci. 2016, 497, 162–171. [Google Scholar] [CrossRef]
- Park, H.; Lee, W.; Park, C. Environmentally Friendly Polymer Hybrids. J. Mater. Sci. 2003, 38, 909–915. [Google Scholar] [CrossRef]
- Sinharay, S.; Bousmina, M. Biodegradable Polymers and Their Layered Silicate Nanocomposites: In Greening the 21st Century Materials World. Prog. Mater. Sci. 2005, 50, 962–1079. [Google Scholar] [CrossRef]
- Llanos, J.H.R.; Tadini, C.C. Preparation and Characterization of Bio-Nanocomposite Films Based on Cassava Starch or Chitosan, Reinforced with Montmorillonite or Bamboo Nanofibers. Int. J. Biol. Macromol. 2018, 107, 371–382. [Google Scholar] [CrossRef]
- Huang, M.; Yu, J.; Ma, X. High Mechanical Performance MMT-Urea and Formamide-Plasticized Thermoplastic Cornstarch Biodegradable Nanocomposites. Carbohydr. Polym. 2006, 63, 393–399. [Google Scholar] [CrossRef]
- Mbey, J.A.; Hoppe, S.; Thomas, F. Cassava Starch–Kaolinite Composite Film. Effect of Clay Content and Clay Modification on Film Properties. Carbohydr. Polym. 2012, 88, 213–222. [Google Scholar] [CrossRef]
- Aimé Mbey, J.; Hoppe, S.; Thomas, F. Cassava Starch-Kaolinite Composite Films. Thermal and Mechanical Properties Related to Filler-Matrix Interactions. Polym. Compos. 2015, 36, 184–191. [Google Scholar] [CrossRef]
- Patakfalvi, R.; Oszkó, A.; Dékány, I. Synthesis and Characterization of Silver Nanoparticle/Kaolinite Composites. Colloids Surf. A Physicochem. Eng. Asp. 2003, 220, 45–54. [Google Scholar] [CrossRef]
- Girdthep, S.; Worajittiphon, P.; Molloy, R.; Lumyong, S.; Leejarkpai, T.; Punyodom, W. Biodegradable Nanocomposite Blown Fi Lms Based on Poly (Lactic Acid) Containing Silver-Loaded Kaolinite: A Route to Controlling Moisture Barrier Property and Silver Ion Release with a Prediction of Extended Shelf Life of Dried Longan. Polymer 2014, 55, 6776–6788. [Google Scholar] [CrossRef]
- Ren, J.; Dang, K.; Pollet, E.; Avérous, L. Preparation and Characterization of Thermoplastic Potato Starch/Halloysite Nano-Biocomposites: Effect of Plasticizer Nature and Nanoclay Content. Polymers 2018, 10, 808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peponi, L.; Puglia, D.; Torre, L.; Valentini, L.; Kenny, J.M. Processing of Nanostructured Polymers and Advanced Polymeric Based Nanocomposites. Mater. Sci. Eng. R Rep. 2014, 85, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A Review on Green Synthesis of Silver Nanoparticles and Their Applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Ghaffari-Moghaddam, M.; Hadi-Dabanlou, R.; Khajeh, M.; Rakhshanipour, M.; Shameli, K. Green Synthesis of Silver Nanoparticles Using Plant Extracts. Korean J. Chem. Eng. 2014, 31, 548–557. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A Review on Plants Extract Mediated Synthesis of Silver Nanoparticles for Antimicrobial Applications: A Green Expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Klueh, U.; Wagner, V.; Kelly, S.; Johnson, A.; Bryers, J.D. Efficacy of Silver-Coated Fabric to Prevent Bacterial Colonization and Subsequent Device-Based Biofilm Formation. J. Biomed. Mater. Res. 2000, 53, 621–631. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; de Carvalho, A.P.A.D.; Conte-Junior, C.A. Recent Advances in Biobased and Biodegradable Polymer Nanocomposites, Nanoparticles, and Natural Antioxidants for Antibacterial and Antioxidant Food Packaging Applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3673–3716. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; Wang, J.; Dong, M.; Jia, P.; Bu, T.; Wang, Q.; Wang, L. Sodium Alginate-Based Nanocomposite Films with Strong Antioxidant and Antibacterial Properties Enhanced by Polyphenol-Rich Kiwi Peel Extracts Bio-Reduced Silver Nanoparticles. Food Packag. Shelf Life 2021, 29, 100741. [Google Scholar] [CrossRef]
- Hassan, A.H.A.; Omnia, A.; Ahmed, A.M.; Khalafalla, F.A.; Ali, F.H.M. Correspondence Antimicrobial Activity of Starch-Based Biodegradable Antimicrobial Films Incorporated with Biosynthesized Silver Nanoparticles Against Multiple Drug-Resistant Staphylococcus Aureus Food Isolates. J. Adv. Vet. Res. 2011, 13, 41–46. [Google Scholar]
- Mohseni, M.S.; Khalilzadeh, M.A.; Mohseni, M.; Hargalani, F.Z.; Getso, M.I.; Raissi, V.; Raiesi, O. Green Synthesis of Ag Nanoparticles from Pomegranate Seeds Extract and Synthesis of Ag-Starch Nanocomposite and Characterization of Mechanical Properties of the Films. Biocatal. Agric. Biotechnol. 2020, 25, 101569. [Google Scholar] [CrossRef]
- Khurshid, S.; Arif, S.; Ali, T.M.; Iqbal, H.M.; Shaikh, M.; Khurshid, H.; Akber, Q.; Yousaf, S. Effect of Silver Nanoparticles Prepared from Saraca Asoca Leaf Extract on Morphological, Functional, Mechanical, and Antibacterial Properties of Rice Starch Films. Starch-Stärke 2022, 74, 2100228. [Google Scholar] [CrossRef]
- Nechita, P. Active-Antimicrobial Coatings Based on Silver Nano-Particles and Natural Polymers for Paper Packaging Functionalization. Nord. Pulp Pap. Res. J. 2017, 32, 452–458. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Starch/Agar-Based Functional Films Integrated with Enoki Mushroom-Mediated Silver Nanoparticles for Active Packaging Applications. Food Biosci. 2022, 49, 101867. [Google Scholar] [CrossRef]
- Rafeeq, C.M.; Paul, E.; Vidya Saagar, E.; Manzur Ali, P.P. Mycosynthesis of Zinc Oxide Nanoparticles Using Pleurotus Floridanus and Optimization of Process Parameters. Ceram. Int. 2021, 47, 12375–12380. [Google Scholar] [CrossRef]
- Yadav, N.; Singh, S.; Saini, O.; Srivastava, S. Technological Advancement in the Remediation of Heavy Metals Employing Engineered Nanoparticles: A Step towards Cleaner Water Process. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100757. [Google Scholar] [CrossRef]
- Kim, S.; Song, K. Bin Antimicrobial Activity of Buckwheat Starch Films Containing Zinc Oxide Nanoparticles against Listeria Monocytogenes on Mushrooms. Int. J. Food Sci. Technol. 2018, 53, 1549–1557. [Google Scholar] [CrossRef]
- Anugrahwidya, R.; Armynah, B.; Tahir, D. Composites Bioplastic Film for Various Concentration of Zinc Oxide (ZnO) Nanocrystals Towards Physical Properties for High Biodegradability in Soil and Seawater. J. Polym. Environ. 2022, 30, 2589–2601. [Google Scholar] [CrossRef]
- Babapour, H.; Jalali, H.; Mohammadi Nafchi, A. The Synergistic Effects of Zinc Oxide Nanoparticles and Fennel Essential Oil on Physicochemical, Mechanical, and Antibacterial Properties of Potato Starch Films. Food Sci. Nutr. 2021, 9, 3893–3905. [Google Scholar] [CrossRef]
- Ni, S.; Zhang, H.; Dai, H.; Xiao, H. Starch-Based Flexible Coating for Food Packaging Paper with Exceptional Hydrophobicity and Antimicrobial Activity. Polymers 2018, 10, 1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosman, N.F.; Nurfazianawatie, M.Z.; Omar, H.; Malek, N.S.A.; Hajar, N.; Buniyamin, I.; Abdullah, S.; Rusop, M.; Asli, N.A. Fungal Growth Physicochemical Properties Inhibition by Novel Zinc Oxide/Glutinous Tapioca Starch Composite. J. Adv. Res. Appl. Sci. Eng. Technol. 2022, 29, 76–89. [Google Scholar] [CrossRef]
- Solís-Gómez, A.; Neira-Velázquez, M.G.; Morales, J.; Sánchez-Castillo, M.A.; Pérez, E. Improving Stability of TiO2 Particles in Water by RF-Plasma Polymerization of Poly(Acrylic Acid) on the Particle Surface. Colloids Surf. A Physicochem. Eng. Asp. 2014, 451, 66–74. [Google Scholar] [CrossRef]
- Vallejo-Montesinos, J.; Gámez-Cordero, J.; Zarraga, R.; Pérez Pérez, M.C.; Gonzalez-Calderon, J.A. Influence of the Surface Modification of Titanium Dioxide Nanoparticles TiO2 under Efficiency of Silver Nanodots Deposition and Its Effect under the Properties of Starch–Chitosan (SC) Films. Polym. Bull. 2020, 77, 107–133. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of Chitosan-TiO2 Composite Film with Efficient Antimicrobial Activities under Visible Light for Food Packaging Applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef]
- Othman, S.H.; Abd Salam, N.R.; Zainal, N.; Kadir Basha, R.; Talib, R.A. Antimicrobial Activity of TiO2 Nanoparticle-Coated Film for Potential Food Packaging Applications. Int. J. Photoenergy 2014, 2014, 945930. [Google Scholar] [CrossRef] [Green Version]
- Tunma, S. Starch Based Nanocomposites in Active Packaging for Extended Shelf Life of Fresh Fruits. Walailak J. Sci. Technol. 2018, 15, 273–281. [Google Scholar] [CrossRef]
- Ostafińska, A.; Mikešová, J.; Krejčíková, S.; Nevoralová, M.; Šturcová, A.; Zhigunov, A.; Michálková, D.; Šlouf, M. Thermoplastic Starch Composites with TiO2 Particles: Preparation, Morphology, Rheology and Mechanical Properties. Int. J. Biol. Macromol. 2017, 101, 273–282. [Google Scholar] [CrossRef]
- Arezoo, E.; Mohammadreza, E.; Maryam, M.; Abdorreza, M.N. The Synergistic Effects of Cinnamon Essential Oil and Nano TiO2 on Antimicrobial and Functional Properties of Sago Starch Films. Int. J. Biol. Macromol. 2020, 157, 743–751. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Harnkarnsujarit, N.; Chongcharoenyanon, B.; Kwon, S.; Ko, S. Enhanced Properties of PBAT/TPS Biopolymer Blend with CuO Nanoparticles for Promising Active Packaging. Food Packag. Shelf Life 2023, 37, 101072. [Google Scholar] [CrossRef]
- Almasi, H.; Jafarzadeh, P.; Mehryar, L. Fabrication of Novel Nanohybrids by Impregnation of CuO Nanoparticles into Bacterial Cellulose and Chitosan Nanofibers: Characterization, Antimicrobial and Release Properties. Carbohydr. Polym. 2018, 186, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.V.; Thaliyakattil, S.; Cherian, L.; Sood, N.; Gokhale, T. Metallic Nanoparticle Integrated Ternary Polymer Blend of PVA/Starch/Glycerol: A Promising Antimicrobial Food Packaging Material. Polymers 2022, 14, 1379. [Google Scholar] [CrossRef]
- Kubo, A.L.; Rausalu, K.; Savest, N.; Žusinaite, E.; Vasiliev, G.; Viirsalu, M.; Plamus, T.; Krumme, A.; Merits, A.; Bondarenko, O. Antibacterial and Antiviral Effects of Ag, Cu and Zn Metals, Respective Nanoparticles and Filter Materials Thereof against Coronavirus SARS-CoV-2 and Influenza A Virus. Pharmaceutics 2022, 14, 2549. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Yin, S.; Dai, Y.; Zhao, J.; Wang, Z.; Xing, B. Antagonism Toxicity of CuO Nanoparticles and Mild Ocean Acidification to Marine Algae. J. Hazard. Mater. 2023, 448, 130857. [Google Scholar] [CrossRef]
- Ramyadevi, J.; Jeyasubramanian, K.; Marikani, A.; Rajakumar, G.; Rahuman, A.A. Synthesis and Antimicrobial Activity of Copper Nanoparticles. Mater. Lett. 2012, 71, 114–116. [Google Scholar] [CrossRef]
- Garcia, C.V.; Shin, G.H.; Kim, J.T. Metal Oxide-Based Nanocomposites in Food Packaging: Applications, Migration, and Regulations. Trends Food Sci. Technol. 2018, 82, 21–31. [Google Scholar] [CrossRef]
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. Evaluation of Antioxidant and Anticancer Activity of Copper Oxide Nanoparticles Synthesized Using Medicinally Important Plant Extracts. Biomed. Pharmacother. 2017, 89, 1067–1077. [Google Scholar] [CrossRef]
- Revathi, T.; Thambidurai, S. Cytotoxic, Antioxidant and Antibacterial Activities of Copper Oxide Incorporated Chitosan-Neem Seed Biocomposites. Int. J. Biol. Macromol. 2019, 139, 867–878. [Google Scholar] [CrossRef]
- Jadhav, M.S.; Kulkarni, S.; Raikar, P.; Barretto, D.A.; Vootla, S.K.; Raikar, U.S. Green Biosynthesis of CuO & Ag-CuO Nanoparticles from Malus Domestica Leaf Extract and Evaluation of Antibacterial, Antioxidant and DNA Cleavage Activities. New J. Chem. 2018, 42, 204–213. [Google Scholar] [CrossRef]
- Das, D.; Nath, B.C.; Phukon, P.; Dolui, S.K. Synthesis and Evaluation of Antioxidant and Antibacterial Behavior of CuO Nanoparticles. Colloids Surf. B Biointerfaces 2013, 101, 430–433. [Google Scholar] [CrossRef]
- Hosseini, S.N.; Pirsa, S.; Farzi, J. Biodegradable Nano Composite Film Based on Modified Starch-Albumin/MgO.; Antibacterial, Antioxidant and Structural Properties. Polym. Test. 2021, 97, 107182. [Google Scholar] [CrossRef]
- Luo, D.; Xie, Q.; Gu, S.; Xue, W. Potato Starch Films by Incorporating Tea Polyphenol and MgO Nanoparticles with Enhanced Physical, Functional and Preserved Properties. Int. J. Biol. Macromol. 2022, 221, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Sani, I.K.; Geshlaghi, S.P.; Pirsa, S.; Asdagh, A. Composite Film Based on Potato Starch/Apple Peel Pectin/ZrO2 Nanoparticles/Microencapsulated Zataria Multiflora Essential Oil; Investigation of Physicochemical Properties and Use in Quail Meat Packaging. Food Hydrocoll. 2021, 117, 106719. [Google Scholar] [CrossRef]
- Sun, Q. Starch Nanoparticles. In Starch in Food; Elsevier: Amsterdam, The Netherlands, 2018; pp. 691–745. ISBN 9780081008683. [Google Scholar]
- Lin, N.; Tang, J.; Dufresne, A.; Tam, M.K.C. (Eds.) Advanced Functional Materials from Nanopolysaccharides; Springer Series in Biomaterials Science and Engineering; Springer: Singapore, 2019; Volume 15, ISBN 978-981-15-0912-4. [Google Scholar]
- Minakawa, A.F.K.; Faria-Tischer, P.C.S.; Mali, S. Simple Ultrasound Method to Obtain Starch Micro- and Nanoparticles from Cassava, Corn and Yam Starches. Food Chem. 2019, 283, 11–18. [Google Scholar] [CrossRef]
- Liu, C.; Li, K.; Li, X.; Zhang, M.; Li, J. Formation and Structural Evolution of Starch Nanocrystals from Waxy Maize Starch and Waxy Potato Starch. Int. J. Biol. Macromol. 2021, 180, 625–632. [Google Scholar] [CrossRef]
- Le Corre, D.; Angellier-Coussy, H. Preparation and Application of Starch Nanoparticles for Nanocomposites: A Review. React. Funct. Polym. 2014, 85, 97–120. [Google Scholar] [CrossRef]
- Bel Haaj, S.; Thielemans, W.; Magnin, A.; Boufi, S. Starch Nanocrystals and Starch Nanoparticles from Waxy Maize as Nanoreinforcement: A Comparative Study. Carbohydr. Polym. 2016, 143, 310–317. [Google Scholar] [CrossRef]
- Angellier, H.; Choisnard, L.; Molina-Boisseau, S.; Ozil, P.; Dufresne, A. Optimization of the Preparation of Aqueous Suspensions of Waxy Maize Starch Nanocrystals Using a Response Surface Methodology. Biomacromolecules 2004, 5, 1545–1551. [Google Scholar] [CrossRef]
- Zhou, J.; Tong, J.; Su, X.; Ren, L. Hydrophobic Starch Nanocrystals Preparations through Crosslinking Modification Using Citric Acid. Int. J. Biol. Macromol. 2016, 91, 1186–1193. [Google Scholar] [CrossRef]
- Wang, S.; Blazek, J.; Gilbert, E.; Copeland, L. New Insights on the Mechanism of Acid Degradation of Pea Starch. Carbohydr. Polym. 2012, 87, 1941–1949. [Google Scholar] [CrossRef]
- Dufresne, A.; Cavaillé, J.-Y.; Helbert, W. New Nanocomposite Materials: Microcrystalline Starch Reinforced Thermoplastic. Macromolecules 1996, 29, 7624–7626. [Google Scholar] [CrossRef]
- Putaux, J.L.; Molina-Boisseau, S.; Momaur, T.; Dufresne, A. Platelet Nanocrystals Resulting from the Disruption of Waxy Maize Starch Granules by Acid Hydrolysis. Biomacromolecules 2003, 4, 1198–1202. [Google Scholar] [CrossRef]
- Dufresne, A. Polysaccharide Nanocrystal Reinforced Nanocomposites. Can. J. Chem. 2008, 86, 484–494. [Google Scholar] [CrossRef]
- LeCorre, D.; Bras, J.; Dufresne, A. Influence of Botanic Origin and Amylose Content on the Morphology of Starch Nanocrystals. J. Nanopart. Res. 2011, 13, 7193–7208. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, D.J.; Kim, J.Y.; Lim, S.T. Preparation of Crystalline Starch Nanoparticles Using Cold Acid Hydrolysis and Ultrasonication. Carbohydr. Polym. 2013, 98, 295–301. [Google Scholar] [CrossRef]
- Sun, Q.; Li, G.; Dai, L.; Ji, N.; Xiong, L. Green Preparation and Characterisation of Waxy Maize Starch Nanoparticles through Enzymolysis and Recrystallisation. Food Chem. 2014, 162, 223–228. [Google Scholar] [CrossRef]
- Bel Haaj, S.; Magnin, A.; Pétrier, C.; Boufi, S. Starch Nanoparticles Formation via High Power Ultrasonication. Carbohydr. Polym. 2013, 92, 1625–1632. [Google Scholar] [CrossRef]
- Patel, C.M.; Chakraborty, M.; Murthy, Z.V.P. Fast and Scalable Preparation of Starch Nanoparticles by Stirred Media Milling. Adv. Powder Technol. 2016, 27, 1287–1294. [Google Scholar] [CrossRef]
- Lamanna, M.; Morales, N.J.; Garcia, N.L.; Goyanes, S. Development and Characterization of Starch Nanoparticles by Gamma Radiation: Potential Application as Starch Matrix Filler. Carbohydr. Polym. 2013, 97, 90–97. [Google Scholar] [CrossRef]
- Liu, D.; Wu, Q.; Chen, H.; Chang, P.R. Transitional Properties of Starch Colloid with Particle Size Reduction from Micro- to Nanometer. J. Colloid Interface Sci. 2009, 339, 117–124. [Google Scholar] [CrossRef]
- Apostolidis, E.; Mandala, I. Modification of Resistant Starch Nanoparticles Using High-Pressure Homogenization Treatment. Food Hydrocoll. 2020, 103, 105677. [Google Scholar] [CrossRef]
- Harsanto, B.W.; Pranoto, Y.; Supriyanto; Kartini, I. Breadfruit-Based Starch Nanoparticles Prepared Using Nanoprecipitation to Stabilize a Pickering Emulsion. J. Southwest Jiaotong Univ. 2021, 56, 372–383. [Google Scholar] [CrossRef]
- Bernardo, C.N.; Kling, I.C.S.; Ferreira, W.H.; Andrade, C.T.; Simao, R.A. Starch Films Containing Starch Nanoparticles as Produced in a Single Step Green Route. Ind. Crops Prod. 2022, 177, 114481. [Google Scholar] [CrossRef]
- Mohammad Amini, A.; Razavi, S.M.A. A Fast and Efficient Approach to Prepare Starch Nanocrystals from Normal Corn Starch. Food Hydrocoll. 2016, 57, 132–138. [Google Scholar] [CrossRef]
- Maryam; Kasim, A.; Novelina; Emriadi. Preparation and Characterization of Sago (Metroxylon Sp.) Starch Nanoparticles Using Hydrolysis-Precipitation Method. J. Phys. Conf. Ser. 2020, 1481, 012021. [Google Scholar] [CrossRef]
- Dai, L.; Li, C.; Zhang, J.; Cheng, F. Preparation and Characterization of Starch Nanocrystals Combining Ball Milling with Acid Hydrolysis. Carbohydr. Polym. 2018, 180, 122–127. [Google Scholar] [CrossRef]
- Shang, Y.; Chao, C.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. Starch Spherulites Prepared by a Combination of Enzymatic and Acid Hydrolysis of Normal Corn Starch. J. Agric. Food Chem. 2018, 66, 6357–6363. [Google Scholar] [CrossRef]
- Yai, H. Mechanical, Thermal and Structural Properties of Rice Starch Films Reinforced with Rice Starch Nanocrystals. Int. Food Res. J. 2013, 20, 439–449. [Google Scholar]
- Oliveira, A.V.; da Silva, A.P.M.; Barros, M.O.; de sá, M.; Souza Filho, M.; Rosa, M.F.; Azeredo, H.M.C. Nanocomposite Films from Mango Kernel or Corn Starch with Starch Nanocrystals. Starch-Staerke 2018, 70, 1800028. [Google Scholar] [CrossRef]
- Xu, C.; Chen, C.; Wu, D. The Starch Nanocrystal Fi Lled Biodegradable Poly (ε-Caprolactone) Composite Membrane with Highly Improved Properties. Carbohydr. Polym. 2018, 182, 115–122. [Google Scholar] [CrossRef]
- Ma, X.; Jian, R.; Chang, P.R.; Yu, J. Fabrication and Characterization of Citric Acid-Modified Starch Nanoparticles/Plasticized-Starch Composites. Biomacromolecules 2008, 9, 3314–3320. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Shanks, R.A. Chemistry and Structure of Cellulosic Fibres as Reinforcements in Natural Fibre Composites. In Natural Fibre Composites; Elsevier: Amsterdam, The Netherlands, 2014; pp. 66–83. ISBN 9780857099228. [Google Scholar]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Gregory, D.A.; Tripathi, L.; Fricker, A.T.R.; Asare, E.; Orlando, I.; Raghavendran, V.; Roy, I. Materials Science & Engineering R Bacterial Cellulose: A Smart Biomaterial with Diverse Applications. Mater. Sci. Eng. R 2021, 145, 100623. [Google Scholar] [CrossRef]
- ISO/TS 20477; Nanotechnologies—Vocabulary for Cellulose Nanomaterial. ISO: Vernier, Switzerland, 2023; Volume 2023.
- Kumar, A.; Dixit, K.; Sinha, N. Fabrication and Characterization of Additively Manufactured CNT-Bioglass Composite Scaffolds Coated with Cellulose Nanowhiskers for Bone Tissue Engineering. Ceram. Int. 2023, 49, 17639–17649. [Google Scholar] [CrossRef]
- Yusefi, M.; Shameli, K.; Lee-Kiun, M.S.; Teow, S.Y.; Moeini, H.; Ali, R.R.; Kia, P.; Jie, C.J.; Abdullah, N.H. Chitosan Coated Magnetic Cellulose Nanowhisker as a Drug Delivery System for Potential Colorectal Cancer Treatment. Int. J. Biol. Macromol. 2023, 233, 123388. [Google Scholar] [CrossRef]
- Sayyed, A.J.; Pinjari, D.V.; Sonawane, S.H.; Bhanvase, B.A.; Sheikh, J.; Sillanpää, M. Cellulose-Based Nanomaterials for Water and Wastewater Treatments: A Review. J. Environ. Chem. Eng. 2021, 9, 106626. [Google Scholar] [CrossRef]
- Aramwit, P.; Bang, N. The Characteristics of Bacterial Nanocellulose Gel Releasing Silk Sericin for Facial Treatment. BMC Biotechnol. 2014, 14, 104. [Google Scholar] [CrossRef] [Green Version]
- Moon, R.J.; Schueneman, G.T.; Simonsen, J. Overview of Cellulose Nanomaterials, Their Capabilities and Applications. JOM 2016, 68, 2383–2394. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different Preparation Methods and Properties of Nanostructured Cellulose from Various Natural Resources and Residues: A Review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Michelin, M.; Gomes, D.G.; Romaní, A.; Polizeli, M.D.L.T.M.; Teixeira, J.A. Nanocellulose Production: Exploring the Enzymatic Route and Residues of Pulp and Paper Industry. Molecules 2020, 25, 3411. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem.—Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, S.J. Cellulose Nanowhiskers: Promising Materials for Advanced Applications. Soft Matter 2011, 7, 303–315. [Google Scholar] [CrossRef]
- Liu, A.; Wu, H.; Naeem, A.; Du, Q.; Ni, B.; Liu, H.; Li, Z.; Ming, L. Cellulose Nanocrystalline from Biomass Wastes: An Overview of Extraction, Functionalization and Applications in Drug Delivery. Int. J. Biol. Macromol. 2023, 241, 124557. [Google Scholar] [CrossRef] [PubMed]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of Nanocrystalline Cellulose from Lignocellulosic Biomass: Technology and Applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef]
- Chang, C.; Hou, J.; Chang, P.R.; Huang, J. Structure and Properties of Cellulose Nanocrystals. In Nanocellulose: From Fundamentals to Advanced Materials; Wiley: Hoboken, NJ, USA, 2019; pp. 21–52. ISBN 9781618394408. [Google Scholar]
- Nessi, V.; Falourd, X.; Maigret, J.E.; Cahier, K.; D’Orlando, A.; Descamps, N.; Gaucher, V.; Chevigny, C.; Lourdin, D. Cellulose Nanocrystals-Starch Nanocomposites Produced by Extrusion: Structure and Behavior in Physiological Conditions. Carbohydr. Polym. 2019, 225, 115123. [Google Scholar] [CrossRef]
- Sucinda, E.F.; Abdul Majid, M.S.; Ridzuan, M.J.M.; Cheng, E.M.; Alshahrani, H.A.; Mamat, N. Development and Characterisation of Packaging Film from Napier Cellulose Nanowhisker Reinforced Polylactic Acid (PLA) Bionanocomposites. Int. J. Biol. Macromol. 2021, 187, 43–53. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in Bio-Based Food Packaging Applications. Ind. Crops Prod. 2017, 97, 664–671. [Google Scholar] [CrossRef] [Green Version]
- Trinh, B.M.; Smith, M.; Mekonnen, T.H. A Nanomaterial-Stabilized Starch-Beeswax Pickering Emulsion Coating to Extend Produce Shelf-Life. Chem. Eng. J. 2022, 431, 133905. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, L.; Zou, J.; Gao, X. The Preparation and Characterization of Nanocomposite Film Reinforced by Modified Cellulose Nanocrystals. Int. J. Biol. Macromol. 2019, 132, 1155–1162. [Google Scholar] [CrossRef]
- Dhali, K.; Daver, F.; Cass, P.; Adhikari, B. Surface Modification of the Cellulose Nanocrystals through Vinyl Silane Grafting. Int. J. Biol. Macromol. 2022, 200, 397–408. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, M.; Cheng, F.; Lin, Y.; Zhu, P.; Li, J.; Tang, K. Preparation and Characterization of Starch-Based Nanocomposites Reinforced by Graphene Oxide Self-Assembled on the Surface of Silane Coupling Agent Modified Cellulose Nanocrystals. Int. J. Biol. Macromol. 2022, 198, 187–193. [Google Scholar] [CrossRef]
- Spence, K.L.; Venditti, R.A.; Rojas, O.J.; Habibi, Y.; Pawlak, J.J. A Comparative Study of Energy Consumption and Physical Properties of Microfibrillated Cellulose Produced by Different Processing Methods. Cellulose 2011, 18, 1097–1111. [Google Scholar] [CrossRef]
- Rol, F.; Karakashov, B.; Nechyporchuk, O.; Terrien, M.; Meyer, V.; Dufresne, A.; Belgacem, M.N.; Bras, J. Pilot-Scale Twin Screw Extrusion and Chemical Pretreatment as an Energy-Efficient Method for the Production of Nanofibrillated Cellulose at High Solid Content. ACS Sustain. Chem. Eng. 2017, 5, 6524–6531. [Google Scholar] [CrossRef]
- Hassan, M.L.; Bras, J.; Hassan, E.A.; Silard, C.; Mauret, E. Enzyme-Assisted Isolation of Microfibrillated Cellulose from Date Palm Fruit Stalks. Ind. Crops Prod. 2014, 55, 102–108. [Google Scholar] [CrossRef]
- Janardhnan, S.; Sain, M.M. Isolation of Cellulose Microfibrils—An Enzymatic Approach. Bioresources 2006, 1, 176–188. [Google Scholar] [CrossRef]
- Eyholzer, C.; Bordeanu, N.; Lopez-Suevos, F.; Rentsch, D.; Zimmermann, T.; Oksman, K. Preparation and Characterization of Water-Redispersible Nanofibrillated Cellulose in Powder Form. Cellulose 2010, 17, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef]
- Hassan, M.L.; Bras, J.; Mauret, E.; Fadel, S.M.; Hassan, E.A.; El-wakil, N.A. Palm Rachis Microfibrillated Cellulose and Oxidized-Microfibrillated Cellulose for Improving Paper Sheets Properties of Unbeaten Softwood and Bagasse Pulps. Ind. Crops Prod. 2015, 64, 9–15. [Google Scholar] [CrossRef]
- Pennells, J.; Godwin, I.D.; Martin, D.J. Trends in the Production of Cellulose Nanofibers from Non- Wood Sources. Cellulose 2020, 27, 575–593. [Google Scholar] [CrossRef]
- Khawas, P.; Deka, S.C. Isolation and Characterization of Cellulose Nanofibers from Culinary Banana Peel Using High-Intensity Ultrasonication Combined with Chemical Treatment. Carbohydr. Polym. 2016, 137, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, G.; Oksman, K.; Tadokoro, S.K.; Mathew, A.P. Re-Dispersible Carrot Nano Fi Bers with High Mechanical Properties and Reinforcing Capacity for Use in Composite Materials. Compos. Sci. Technol. 2016, 123, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Balakrishnan, P.; Gopi, S.; MS, S.; Thomas, S. UV Resistant Transparent Bionanocomposite Films Based on Potato Starch/Cellulose for Sustainable Packaging. Starch-Stärke 2018, 70, 1700139. [Google Scholar] [CrossRef]
- Fazeli, M.; Keley, M.; Biazar, E. Preparation and Characterization of Starch-Based Composite Films Reinforced by Cellulose Nanofibers. Int. J. Biol. Macromol. 2018, 116, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Hietala, M.; Mathew, A.P.; Oksman, K. Bionanocomposites of Thermoplastic Starch and Cellulose Nanofibers Manufactured Using Twin-Screw Extrusion. Eur. Polym. J. 2013, 49, 950–956. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y.; Chen, G. A Comparative Study on the Starch-Based Biocomposite Films Reinforced by Nanocellulose Prepared from Different Non-Wood Fibers. Cellulose 2019, 26, 2425–2435. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, J.; Zhang, Y.; Li, F.; Jiao, X.; Li, Q. The Effects of Cellulose Nanocrystal and Cellulose Nanofiber on the Properties of Pumpkin Starch-Based Composite Films. Int. J. Biol. Macromol. 2021, 192, 444–451. [Google Scholar] [CrossRef]
- Punia, S.; Scott, W.; Ozogul, F.; Dunno, K.D.; Armstrong, G.; Dawson, P. Food Bioscience Development of Starch-Based Films Reinforced with Cellulosic Nanocrystals and Essential Oil to Extend the Shelf Life of Red Grapes. Food Biosci. 2022, 47, 101621. [Google Scholar] [CrossRef]
- Kümmerer, K.; Menz, J.; Schubert, T.; Thielemans, W. Biodegradability of Organic Nanoparticles in the Aqueous Environment. Chemosphere 2011, 82, 1387–1392. [Google Scholar] [CrossRef]
- Ullah, H.; Santos, H.A.; Khan, T. Applications of Bacterial Cellulose in Food, Cosmetics and Drug Delivery. Cellulose 2016, 23, 2291–2314. [Google Scholar] [CrossRef]
- Dourado, F.; Gama, M.; Rodrigues, A.C. A Review on the Toxicology and Dietetic Role of Bacterial Cellulose. Toxicol. Rep. 2017, 4, 543–553. [Google Scholar] [CrossRef] [Green Version]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial Cellulose as a Raw Material for Food and Food Packaging Applications. Front. Sustain. Food Syst. 2019, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of Bacterial Cellulose in Food. Food Hydrocoll. 2014, 35, 539–545. [Google Scholar] [CrossRef]
- Mohammadkazemi, F.; Azin, M.; Ashori, A. Production of Bacterial Cellulose Using Different Carbon Sources and Culture Media. Carbohydr. Polym. 2015, 117, 518–523. [Google Scholar] [CrossRef]
- Avcioglu, N.H. Bacterial Cellulose: Recent Progress in Production and Industrial Applications. World J. Microbiol. Biotechnol. 2022, 38, 86. [Google Scholar] [CrossRef]
- Esa, F.; Tasirin, S.M.; Rahman, N.A. Overview of Bacterial Cellulose Production and Application. Agric. Agric. Sci. Procedia 2014, 2, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Fabra, M.J.; López-Rubio, A.; Ambrosio-Martín, J.; Lagaron, J.M. Improving the Barrier Properties of Thermoplastic Corn Starch-Based Films Containing Bacterial Cellulose Nanowhiskers by Means of PHA Electrospun Coatings of Interest in Food Packaging. Food Hydrocoll. 2016, 61, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Grande, C.J.; Torres, F.G.; Gomez, C.M.; Troncoso, O.P.; Canet-Ferrer, J.; Martínez-Pastor, J. Development of Self-Assembled Bacterial Cellulose-Starch Nanocomposites. Mater. Sci. Eng. C 2009, 29, 1098–1104. [Google Scholar] [CrossRef]
- Paillet, M.; Dufresne, A. Chitin Whisker Reinforced Thermoplastic Nanocomposites. Macromolecules 2001, 34, 6527–6530. [Google Scholar] [CrossRef]
- Muñoz-Núñez, C.; Fernández-García, M.; Muñoz-Bonilla, A. Chitin Nanocrystals: Environmentally Friendly Materials for the Development of Bioactive Films. Coatings 2022, 12, 144. [Google Scholar] [CrossRef]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for Chitosan Based Antimicrobial Films in Food Applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Shapi’i, R.A.; Othman, S.H.; Nordin, N.; Kadir Basha, R.; Nazli Naim, M. Antimicrobial Properties of Starch Films Incorporated with Chitosan Nanoparticles: In Vitro and in Vivo Evaluation. Carbohydr. Polym. 2020, 230, 115602. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.Z.; Zhu, K.J. A Novel Approach to Prepare Tripolyphosphate/Chitosan Complex Beads for Controlled Release Drug Delivery. Int. J. Pharm. 2000, 201, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesàro, A. Chitosan Nanoparticles: Preparation, Size Evolution and Stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef]
- Chang, P.R.; Jian, R.; Yu, J.; Ma, X. Fabrication and Characterisation of Chitosan Nanoparticles/Plasticised-Starch Composites. Food Chem. 2010, 120, 736–740. [Google Scholar] [CrossRef]
- Chang, P.R.; Jian, R.; Yu, J.; Ma, X. Starch-Based Composites Reinforced with Novel Chitin Nanoparticles. Carbohydr. Polym. 2010, 80, 420–425. [Google Scholar] [CrossRef]
- Antoniou, J.; Liu, F.; Majeed, H.; Zhong, F. Characterization of Tara Gum Edible Films Incorporated with Bulk Chitosan and Chitosan Nanoparticles: A Comparative Study. Food Hydrocoll. 2015, 44, 309–319. [Google Scholar] [CrossRef]
- Li, R.; Zhuang, D.; Feng, H.; Wang, S.; Zhu, J. Novel “All-in-One” Multifunctional Gelatin-Based Film for Beef Freshness Maintaining and Monitoring. Food Chem. 2023, 418, 136003. [Google Scholar] [CrossRef]
- McKeen, L.W. Introduction to Plastics and Polymers. In Film Properties of Plastics and Elastomers; William Andrew: Norwich, NY, USA, 2012; pp. 1–18. [Google Scholar] [CrossRef]
- Hazrati, K.Z.; Sapuan, S.M.; Zuhri, M.Y.M.; Jumaidin, R. Effect of Plasticizers on Physical, Thermal, and Tensile Properties of Thermoplastic Films Based on Dioscorea Hispida Starch. Int. J. Biol. Macromol. 2021, 185, 219–228. [Google Scholar] [CrossRef]
- Mali, S.; Sakanaka, L.S.; Yamashita, F.; Grossmann, M.V.E. Water Sorption and Mechanical Properties of Cassava Starch Films and Their Relation to Plasticizing Effect. Carbohydr. Polym. 2005, 60, 283–289. [Google Scholar] [CrossRef]
- Maruddin, F.; Malaka, R.; Baba, S.; Amqam, H.; Taufik, M.; Sabil, S. Brightness, Elongation and Thickness of Edible Film with Caseinate Sodium Using a Type of Plasticizer. IOP Conf. Ser. Earth Environ. Sci. 2020, 492, 012043. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of Plasticizer Type and Concentration on Physical Properties of Biodegradable Films Based on Sugar Palm (Arenga pinnata) Starch for Food Packaging. J. Food Sci. Technol. 2016, 53, 326–336. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Tan, C.; Xie, J.; Yuan, F.; Tao, H.; Guo, L.; Cui, B.; Yuan, C.; Gao, W.; Zou, F.; et al. Effects of Different Ratios of Mannitol to Sorbitol on the Functional Properties of Sweet Potato Starch Films. Int. J. Biol. Macromol. 2023, 242, 124914. [Google Scholar] [CrossRef]
- Chillo, S.; Flores, S.; Mastromatteo, M.; Conte, A.; Gerschenson, L.; Del Nobile, M.A. Influence of Glycerol and Chitosan on Tapioca Starch-Based Edible Film Properties. J. Food Eng. 2008, 88, 159–168. [Google Scholar] [CrossRef]
- Sahari, J.; Sapuan, S.M.; El-Shekeil, Y.A.; Ishak, M.R.; Akhtar, R. Natural Fibre-Reinforced Thermoplastic Starch Composites. In Starch-Based Blends, Composites and Nanocomposites; The Royal Society of Chemistry: London, UK, 2015; pp. 109–142. [Google Scholar]
- Geleta, T.T.; Habtegebreil, S.A.; Tolesa, G.N. Physical, Mechanical, and Optical Properties of Enset Starch from Bulla Films Influenced by Different Glycerol Concentrations and Temperatures. J. Food Process. Preserv. 2020, 44, e14586. [Google Scholar] [CrossRef]
- Ben, Z.Y.; Samsudin, H.; Yhaya, M.F. Glycerol: Its Properties, Polymer Synthesis, and Applications in Starch Based Films. Eur. Polym. J. 2022, 175, 111377. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and Biodegradable Starch Films: A Review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- Zahiruddin, S.M.M.; Othman, S.H.; Tawakkal, I.S.M.A.; Talib, R.A. Mechanical and Thermal Properties of Tapioca Starch Films Plasticized with Glycerol and Sorbitol. Food Res. 2019, 3, 157–163. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Tessaro, L.; Ramos, A.P.; Tapia-Blácido, D.R. Which Plasticizer Is Suitable for Films Based on Babassu Starch Isolated by Different Methods? Food Hydrocoll. 2019, 89, 143–152. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Giordani, C.; Casettari, L.; Curzi, G.; Cappellacci, L.; Petrelli, R.; Maggi, F. Acute and Sub-Lethal Toxicity of Eight Essential Oils of Commercial Interest against the Filariasis Mosquito Culex Quinquefasciatus and the Housefly Musca Domestica. Ind. Crops Prod. 2018, 112, 668–680. [Google Scholar] [CrossRef]
- Angulo-Milhem, S.; Verriele, M.; Nicolas, M.; Thevenet, F. Indoor Use of Essential Oils: Emission Rates, Exposure Time and Impact on Air Quality. Atmos. Environ. 2021, 244, 117863. [Google Scholar] [CrossRef]
- Mishra, A.P.; Devkota, H.P.; Nigam, M.; Adetunji, C.O.; Srivastava, N.; Saklani, S.; Shukla, I.; Azmi, L.; Shariati, M.A.; Melo Coutinho, H.D.; et al. Combination of Essential Oils in Dairy Products: A Review of Their Functions and Potential Benefits. LWT 2020, 133, 110116. [Google Scholar] [CrossRef]
- Mahmood, K.; Kamilah, H.; Karim, A.A.; Ariffin, F. Enhancing the Functional Properties of Fish Gelatin Mats by Dual Encapsulation of Essential Oils in β-Cyclodextrins/Fish Gelatin Matrix via Coaxial Electrospinning. Food Hydrocoll. 2023, 137, 108324. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential Oils as Additives in Active Food Packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Shlosman, K.; Rein, D.M.; Shemesh, R.; Koifman, N.; Caspi, A.; Cohen, Y. Encapsulation of Thymol and Eugenol Essential Oils Using Unmodified Cellulose: Preparation and Characterization. Polymers 2023, 15, 95. [Google Scholar] [CrossRef]
- Detoni, C.B.; De Oliveira, D.M.; Santo, I.E.; Pedro, A.S.; El-Bacha, R.; Da Silva Velozo, E.; Ferreira, D.; Sarmento, B.; De Magalhães Cabral-Albuquerque, E.C. Evaluation of Thermal-Oxidative Stability and Antiglioma Activity of Zanthoxylum tingoassuiba Essential Oil Entrapped into Multi- and Unilamellar Liposomes. J. Liposome Res. 2012, 22, 1–7. [Google Scholar] [CrossRef]
- Shi, F.; Zhao, J.H.; Liu, Y.; Wang, Z.; Zhang, Y.T.; Feng, N.P. Preparation and Characterization of Solid Lipid Nanoparticles Loaded with Frankincense and Myrrh Oil. Int. J. Nanomed. 2012, 7, 2033–2043. [Google Scholar] [CrossRef] [Green Version]
- Loi, M.; Paciolla, C.; Logrieco, A.F.; Mulè, G. Plant Bioactive Compounds in Pre- and Postharvest Management for Aflatoxins Reduction. Front. Microbiol. 2020, 11, 243. [Google Scholar] [CrossRef] [Green Version]
- Castillo, F.; Hernández, D.; Gallegos, G.; Rodríguez, R.; Aguilar, C.N. Antifungal Properties of Bioactive Compounds from Plants; INTECH Open Access Publisher: London, UK, 2012. [Google Scholar]
- Gershenzon, J.; Dudareva, N. The Function of Terpene Natural Products in the Natural World. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Gutiérrez-Del-río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and Polyphenols as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef]
- Lafay, S.; Gil-Izquierdo, A. Bioavailability of Phenolic Acids. Phytochem. Rev. 2008, 7, 301–311. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [Green Version]
- Pateiro, M.; Gómez-Salazar, J.A.; Jaime-Patlán, M.; Sosa-Morales, M.E.; Lorenzo, J.M. Plant Extracts Obtained with Green Solvents as Natural Antioxidants in Fresh Meat Products. Antioxidants 2021, 10, 181. [Google Scholar] [CrossRef]
- Pateiro, M.; Munekata, P.E.S.; Sant’Ana, A.S.; Domínguez, R.; Rodríguez-Lázaro, D.; Lorenzo, J.M. Application of Essential Oils as Antimicrobial Agents against Spoilage and Pathogenic Microorganisms in Meat Products. Int. J. Food Microbiol. 2021, 337, 108966. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Jo, C. Essential Oils as Potential Antimicrobial Agents in Meat and Meat Products: A Review. Trends Food Sci. Technol. 2013, 34, 96–108. [Google Scholar] [CrossRef]
- Fiore, A.; Park, S.; Volpe, S.; Torrieri, E.; Masi, P. Active Packaging Based on PLA and Chitosan-Caseinate Enriched Rosemary Essential Oil Coating for Fresh Minced Chicken Breast Application. Food Packag. Shelf Life 2021, 29, 100708. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Moghaddas Kia, E.; Ghasempour, Z.; Ehsani, A. Preparation of Active Nanocomposite Film Consisting of Sodium Caseinate, ZnO Nanoparticles and Rosemary Essential Oil for Food Packaging Applications. J. Polym. Environ. 2021, 29, 588–598. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Rodrigues, C.; Ferreira, L.; Pires, J.R.A.; Duarte, M.P.; Coelhoso, I.; Fernando, A.L. In Vitro Bioactivity of Novel Chitosan Bionanocomposites Incorporated with Different Essential Oils. Ind. Crops Prod. 2019, 140, 111563. [Google Scholar] [CrossRef]
- Restrepo, A.E.; Rojas, J.D.; García, O.R.; Sánchez, L.T.; Pinzón, M.I.; Villa, C.C. Mechanical, Barrier, and Color Properties of Banana Starch Edible Films Incorporated with Nanoemulsions of Lemongrass (Cymbopogon citratus) and Rosemary (Rosmarinus officinalis) Essential Oils. Food Sci. Technol. Int. 2018, 24, 705–712. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Genipin-Crosslinked Gelatin/Chitosan-Based Functional Films Incorporated with Rosemary Essential Oil and Quercetin. Materials 2022, 15, 3769. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, N.; Fu, Y.J.; Wang, W.; Luo, M.; Zhao, C.J.; Zu, Y.G.; Liu, X.L. Chemical Composition and Antimicrobial Activity of the Essential Oil of Rosemary. Environ. Toxicol. Pharmacol. 2011, 32, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Tafa, T.G.; Engida, A.M. Preparation of Green Film with Improved Physicochemical Properties and Enhanced Antimicrobial Activity Using Ingredients from Cassava Peel, Bamboo Leaf and Rosemary Leaf. Heliyon 2022, 8, e10130. [Google Scholar] [CrossRef] [PubMed]
- Rivera Leiva, A.F.; Hernández-Fernández, J.; Ortega Toro, R. Active Films Based on Starch and Wheat Gluten (Triticum vulgare) for Shelf-Life Extension of Carrots. Polymers 2022, 14, 5077. [Google Scholar] [CrossRef]
- Syafiq, R.; Sapuan, S.M.; Zuhri, M.R.M. Antimicrobial Activity, Physical, Mechanical and Barrier Properties of Sugar Palm Based Nanocellulose/Starch Biocomposite Films Incorporated with Cinnamon Essential Oil. J. Mater. Res. Technol. 2021, 11, 144–157. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, X.; Chen, J.; He, J. Effects of Cinnamon Essential Oil on the Physical, Mechanical, Structural and Thermal Properties of Cassava Starch-Based Edible Films. Int. J. Biol. Macromol. 2021, 184, 574–583. [Google Scholar] [CrossRef]
- Lu, F.; Ding, Y.C.; Ye, X.Q.; Ding, Y.T. Antibacterial Effect of Cinnamon Oil Combined with Thyme or Clove Oil. Agric. Sci. China 2011, 10, 1482–1487. [Google Scholar] [CrossRef]
- Singh, G.; Maurya, S.; de Lampasona, M.P.; Catalan, C.A.N. A Comparison of Chemical, Antioxidant and Anti-microbial Studies of Cinnamon Leaf and Bark Volatile Oils, Oleoresins and Their Constituents. Food Chem. Toxicol. 2007, 45, 1650–1661. [Google Scholar] [CrossRef]
- Trifan, A.; Zengin, G.; Brebu, M.; Skalicka-woźniak, K.; Luca, S.V. Phytochemical Characterization and Evaluation of the Antioxidant and Anti-enzymatic Activity of Five Common Spices: Focus on Their Essential Oils and Spent Material Extractives. Plants 2021, 10, 2692. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Q.; Wang, Z.; Cao, H.; Zhang, D. Structure-Activity Relationships of Cinnamaldehyde and Eugenol Derivatives against Plant Pathogenic Fungi. Ind. Crops Prod. 2017, 97, 388–394. [Google Scholar] [CrossRef]
- Clemente, I.; Aznar, M.; Silva, F.; Nerín, C. Antimicrobial Properties and Mode of Action of Mustard and Cinnamon Essential Oils and Their Combination against Foodborne Bacteria. Innov. Food Sci. Emerg. Technol. 2016, 36, 26–33. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. Antimicrobial Activity of Plant Essential Oils Using Food Model Media: Efficacy, Synergistic Potential and Interactions with Food Components. Food Microbiol. 2009, 26, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G.; Huvaere, K.; Skibsted, L.H. Antioxidant Activity of Rosemary and Thyme By-Products and Synergism with Added Antioxidant in a Liposome System. Eur. Food Res. Technol. 2011, 233, 11–18. [Google Scholar] [CrossRef]
- Cai, C.; Ma, R.; Duan, M.; Deng, Y.; Liu, T.; Lu, D. Effect of Starch Film Containing Thyme Essential Oil Microcapsules on Physicochemical Activity of Mango. LWT 2020, 131, 109700. [Google Scholar] [CrossRef]
- Issa, A.; Ibrahim, S.A.; Tahergorabi, R. Impact of Sweet Potato Starch-Based Nanocomposite Films Activated with Thyme Essential Oil on the Shelf-Life of Baby Spinach Leaves. Foods 2017, 6, 43. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Cheng, M.; Han, M.; Zhang, R.; Wang, X. Characterization and Release Kinetics Study of Potato Starch Nanocomposite Films Containing Mesoporous Nano-Silica Incorporated with Thyme Essential Oil. Int. J. Biol. Macromol. 2021, 184, 566–573. [Google Scholar] [CrossRef]
- Park, S.Y.; Raka, R.N.; Hui, X.L.; Song, Y.; Sun, J.L.; Xiang, J.; Wang, J.; Jin, J.M.; Li, X.K.; Xiao, J.S.; et al. Six Spain Thymus Essential Oils Composition Analysis and Their in Vitro and in Silico Study against Streptococcus Mutans. BMC Complement. Med. Ther. 2023, 23, 106. [Google Scholar] [CrossRef]
- Sivaram, S.; Somanathan, H.; Kumaresan, S.M.; Muthuraman, M.S. The Beneficial Role of Plant Based Thymol in Food Packaging Application: A Comprehensive Review. Appl. Food Res. 2022, 2, 100214. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K.H. Synergistic Antioxidant and Antibacterial Advantages of Essential Oils for Food Packaging Applications. Biomolecules 2021, 11, 1267. [Google Scholar] [CrossRef]
- López-Gómez, A.; Navarro-Martínez, A.; Garre, A.; Iguaz, A.; Maldonado-Guzmán, P.; Martínez-Hernández, G.B. Kinetics of Carvacrol Release from Active Paper Packaging for Fresh Fruits and Vegetables under Conditions of Open and Closed Package. Food Packag. Shelf Life 2023, 37, 101081. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, L.; Zhang, Y.; Huang, Y.; Cao, J.; Jiang, W. Antimicrobial and Controlled Release Properties of Nanocomposite Film Containing Thymol and Carvacrol Loaded UiO-66-NH2 for Active Food Packaging. Food Chem. 2023, 404, 134427. [Google Scholar] [CrossRef]
- El Abdali, Y.; Mahraz, A.M.; Beniaich, G.; Mssillou, I.; Chebaibi, M.; Bin Jardan, Y.A.; Lahkimi, A.; Nafidi, H.A.; Aboul-Soud, M.A.M.; Bourhia, M.; et al. Essential Oils of Origanum Compactum Benth: Chemical Characterization, in Vitro, in Silico, Antioxidant, and Antibacterial Activities. Open Chem. 2023, 21, 20220282. [Google Scholar] [CrossRef]
- Bora, L.; Burkard, T.; Juan, M.H.S.; Radeke, H.H.; Muț, A.M.; Vlaia, L.L.; Magyari-Pavel, I.Z.; Diaconeasa, Z.; Socaci, S.; Borcan, F.; et al. Phytochemical Characterization and Biological Evaluation of Origanum Vulgare L. Essential Oil Formulated as Polymeric Micelles Drug Delivery Systems. Pharmaceutics 2022, 14, 2413. [Google Scholar] [CrossRef]
- Song, X.; Zuo, G.; Chen, F. Effect of Essential Oil and Surfactant on the Physical and Antimicrobial Properties of Corn and Wheat Starch Films. Int. J. Biol. Macromol. 2018, 107, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan Eliuz, E.; Bahadırlı, N.P. Investigation of Antibacterial Activity and Mechanism of T. Spicata Essential Oil, and Activation of the Hydrosol Formed as a by-Product with UV. Biologia 2023, 78, 1161–1170. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Pérez-Pérez, J.C.; Varillas-Torres, J.M.; Navarro-Cruz, A.R.; Hernández-Carranza, P.; Munguía-Pérez, R.; Cid-Pérez, T.S.; Avila-Sosa, R. Starch Edible Films/Coatings Added with Carvacrol and Thymol: In Vitro and in Vivo Evaluation against Colletotrichum Gloeosporioides. Foods 2021, 10, 175. [Google Scholar] [CrossRef]
- Mao, S.; Li, F.; Zhou, X.; Lu, C.; Zhang, T. Characterization and Sustained Release Study of Starch-Based Films Loaded with Carvacrol: A Promising UV-Shielding and Bioactive Nanocomposite Film. LWT 2023, 180, 114719. [Google Scholar] [CrossRef]
- Razzouk, S.; Mazri, M.A.; Jeldi, L.; Mnasri, B.; Ouahmane, L.; Alfeddy, M.N. Chemical Composition and Antimicrobial Activity of Essential Oils from Three Mediterranean Plants against Eighteen Pathogenic Bacteria and Fungi. Pharmaceutics 2022, 14, 1608. [Google Scholar] [CrossRef]
- Kiki, M.J. In Vitro Antiviral Potential, Antioxidant, and Chemical Composition of Clove (Syzygium aromaticum) Essential Oil. Molecules 2023, 28, 2421. [Google Scholar] [CrossRef]
- Devi, K.P.; Sakthivel, R.; Nisha, S.A.; Suganthy, N.; Pandian, S.K. Eugenol Alters the Integrity of Cell Membrane and Acts against the Nosocomial Pathogen Proteus Mirabilis. Arch. Pharm. Res. 2013, 36, 282–292. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an Essential Oil of Clove) Acts as an Antibacterial Agent against Salmonella Typhi by Disrupting the Cellular Membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Chen, H.; Xu, R.; Liu, B.; Yuan, C.; Guo, L.; Liu, P.; Fang, Y.; Cui, B. Green Preparation of 3D Micronetwork Eugenol-Encapsuled Porous Starch for Improving the Performance of Starch-Based Antibacterial Film. Int. J. Biol. Macromol. 2023, 241, 124593. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, F.; Mu, W.; Han, X. Antioxidant and Antibacterial Starch-Based Edible Films Composed of Eugenol/Gelatin Microspheres. New J. Chem. 2023, 47, 4228–4238. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, H.; Kang, S.; Xia, L.; Jiang, S.; Chen, M.; Jiang, S. An Active Packaging Film Based on Yam Starch with Eugenol and Its Application for Pork Preservation. Food Hydrocoll. 2019, 96, 546–554. [Google Scholar] [CrossRef]
- Haddou, S.; Hassania Loukili, E.; Hbika, A.; Chahine, A.; Hammouti, B. Phytochemical Study Using HPLC-UV/GC–MS of Different of Cannabis sativa L. Seeds Extracts from Morocco. Mater. Today Proc. 2023, 72, 3896–3903. [Google Scholar] [CrossRef]
- Benoutman, A.; Erbiai, E.H.; Edderdaki, F.Z.; Cherif, E.K.; Saidi, R.; Lamrani, Z.; Pintado, M.; Pinto, E.; Esteves da Silva, J.C.G.; Maouni, A. Phytochemical Composition, Antioxidant and Antifungal Activity of Thymus Capitatus, a Medicinal Plant Collected from Northern Morocco. Antibiotics 2022, 11, 681. [Google Scholar] [CrossRef]
- Autor, E.; Cornejo, A.; Bimbela, F.; Maisterra, M.; Gandía, L.M.; Martínez-Merino, V. Extraction of Phenolic Compounds from Populus Salicaceae Bark. Biomolecules 2022, 12, 539. [Google Scholar] [CrossRef]
- Anwar, A.; Siddiqui, R.; Shah, M.R.; Khan, N.A. Gold Nanoparticle-Conjugated Cinnamic Acid Exhibits Antiacanthamoebic and Antibacterial Properties. Antimicrob. Agents Chemother. 2018, 62, 10–1128. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, E.; Bail, S.; Friedl, S.M.; Jirovetz, L.; Buchbauer, G.; Wanner, J.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Geissler, M. Antimicrobial Activities of Single Aroma Compounds 1. Nat. Prod. Commun. 2010, 5, 1934578X1000500906. [Google Scholar] [CrossRef] [Green Version]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Antibacterial Properties of Cinnamic and Ferulic Acids Incorporated to Starch and PLA Monolayer and Multilayer Films. Food Control 2022, 136, 108878. [Google Scholar] [CrossRef]
- Che Hamzah, N.H.; Khairuddin, N.; Muhamad, I.I.; Hassan, M.A.; Ngaini, Z.; Sarbini, S.R. Characterisation and Colour Response of Smart Sago Starch-Based Packaging Films Incorporated with Brassica Oleracea Anthocyanin. Membranes 2022, 12, 913. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, R.; Ezati, P.; Rhim, J.-W. Recent Advances in Intelligent Food Packaging Applications Using Natural Food Colorants. ACS Food Sci. Technol. 2021, 1, 124–138. [Google Scholar] [CrossRef]
- Kong, J.; Ge, X.; Sun, Y.; Mao, M.; Yu, H.; Chu, R.; Wang, Y. Multi-Functional PH-Sensitive Active and Intelligent Packaging Based on Highly Cross-Linked Zein for the Monitoring of Pork Freshness. Food Chem. 2023, 404, 134754. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Sun, H.; Wan, X.; Wu, Y.; Lin, X.; Kan, H.; Hou, D.; Zheng, Z.; He, X.; Liu, C. Carboxymethylcellulose Reinforced Starch Films and Rapid Detection of Spoiled Beverages. Front. Bioeng. Biotechnol. 2023, 10, 1099118. [Google Scholar] [CrossRef]
- Huang, H.L.; Tsai, I.L.; Lin, C.; Hang, Y.H.; Ho, Y.C.; Tsai, M.L.; Mi, F.L. Intelligent Films of Marine Polysaccharides and Purple Cauliflower Extract for Food Packaging and Spoilage Monitoring. Carbohydr. Polym. 2023, 299, 120133. [Google Scholar] [CrossRef]
- De Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From Plant to Health. Phytochem. Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Li, L.; Wang, W.; Zheng, M.; Sun, J.; Chen, Z.; Wang, J.; Ma, Q. Nanocellulose-Enhanced Smart Film for the Accurate Monitoring of Shrimp Freshness via Anthocyanin-Induced Color Changes. Carbohydr. Polym. 2023, 301, 120352. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Anthocyanin Food Colorant and Its Application in PH-Responsive Color Change Indicator Films. Crit. Rev. Food Sci. Nutr. 2021, 61, 2297–2325. [Google Scholar] [CrossRef]
- Fotouhi, M.; Nasihatkon, B.; Solouki, S.; Seidi, S. A Card Instead of a Lab: A Ligand Embedded in a Bio-Composite of Starch/Gelatin Intelligent Film for Milk Quality Test Followed by Colorimetric Analysis. Int. J. Biol. Macromol. 2023, 228, 426–434. [Google Scholar] [CrossRef]
- Qin, Y.; Yun, D.; Xu, F.; Chen, D.; Kan, J.; Liu, J. Smart Packaging Films Based on Starch/Polyvinyl Alcohol and Lycium Ruthenicum Anthocyanins-Loaded Nano-Complexes: Functionality, Stability and Application. Food Hydrocoll. 2021, 119, 106850. [Google Scholar] [CrossRef]
- Vedove, T.M.A.R.D.; Maniglia, B.C.; Tadini, C.C. Production of Sustainable Smart Packaging Based on Cassava Starch and Anthocyanin by an Extrusion Process. J. Food Eng. 2021, 289, 110274. [Google Scholar] [CrossRef]
- Zhai, X.; Shi, J.; Zou, X.; Wang, S.; Jiang, C.; Zhang, J.; Huang, X.; Zhang, W.; Holmes, M. Novel Colorimetric Films Based on Starch/Polyvinyl Alcohol Incorporated with Roselle Anthocyanins for Fish Freshness Monitoring. Food Hydrocoll. 2017, 69, 308–317. [Google Scholar] [CrossRef] [Green Version]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent PH Indicator Film Composed of Agar/Potato Starch and Anthocyanin Extracts from Purple Sweet Potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef]
- Zong, Z.; Liu, M.; Chen, H.; Farag, M.A.; Wu, W.; Fang, X.; Niu, B.; Gao, H. Preparation and Characterization of a Novel Intelligent Starch/Gelatin Binary Film Containing Purple Sweet Potato Anthocyanins for Flammulina Velutipes Mushroom Freshness Monitoring. Food Chem. 2023, 405, 134839. [Google Scholar] [CrossRef]
- Ceballos, R.L.; Ochoa-Yepes, O.; Goyanes, S.; Bernal, C.; Famá, L. Effect of Yerba Mate Extract on the Performance of Starch Films Obtained by Extrusion and Compression Molding as Active and Smart Packaging. Carbohydr. Polym. 2020, 244, 116495. [Google Scholar] [CrossRef]
- Kavas, E.; Terzioğlu, P.; Sıcak, Y. Betanin and Nano-Calcium Carbonate Incorporated Polyvinyl Alcohol/Starch Films for Active and Intelligent Packaging Applications. J. Polym. Environ. 2023. [Google Scholar] [CrossRef]
- Naghdi, S.; Rezaei, M.; Abdollahi, M. A Starch-Based PH-Sensing and Ammonia Detector Film Containing Betacyanin of Paperflower for Application in Intelligent Packaging of Fish. Int. J. Biol. Macromol. 2021, 191, 161–170. [Google Scholar] [CrossRef]
- Liu, D.; Dang, S.; Zhang, L.; Munsop, K.; Li, X. Corn Starch/Polyvinyl Alcohol Based Films Incorporated with Curcumin-Loaded Pickering Emulsion for Application in Intelligent Packaging. Int. J. Biol. Macromol. 2021, 188, 974–982. [Google Scholar] [CrossRef]
- Hazer, S.; Aytac, A. Monitoring Food Quality—Effect of Curcumin in the Development of Polyethylene/Thermoplastic Starch Based Smart Packaging. J. Vinyl Addit. Technol. 2023, 1–14. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W. Starch and Agar-Based Color-Indicator Films Integrated with Shikonin for Smart Packaging Application of Shrimp. ACS Food Sci. Technol. 2021, 1, 1963–1969. [Google Scholar] [CrossRef]
- Ranjeth Kumar Reddy, T.; Kim, H.J. Mechanical, Optical, Thermal, and Barrier Properties of Poly (Lactic Acid)/Curcumin Composite Films Prepared Using Twin-Screw Extruder. Food Biophys. 2019, 14, 22–29. [Google Scholar] [CrossRef]















| Corn (Maize) Starch | ||
|---|---|---|
| Content | Amount | Units |
| Density [26,27,28] | 1.356–1.4029 | g/cm3 |
| Amylose [26,27,28,29] | 24.64–29.4 | g/100 g |
| Amylopectin [26,27] | 72–75.36 | g/100 g |
| Crude fats [26,27,28] | 0.32–7.13 | g/100 g |
| Crude proteins [26,27,28] | 7.70–0.38 | g/100 g |
| Moisture contents [26,27,28,29] | 10.45–12.7 | % |
| Potato starch | ||
| Content | Amount | Units |
| Amylose [30,31] | 17–38.8 | g/100 g |
| Amylopectin [32] | 73.42 | g/100 g |
| Crude fats [31] | 0.06–0.20 | g/100 g |
| Crude proteins [31] | 0.01–0.09 | g/100 g |
| Moisture contents [33] | 9–11 | % |
| Rice starch | ||
| Content | Amount | Units |
| Amylose [34,35,36] | 4.1–32 | g/100 g |
| Amylopectin [32] | 74.56 | g/100 g |
| Crude fats [34] | 1.9–28.1 | g/100 g |
| Crude proteins [34] | 0–3.0 | g/100 g |
| Moisture contents [34] | 13 | % |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Gimena, P.F.; Oliver-Cuenca, V.; Peponi, L.; López, D. A Review on Reinforcements and Additives in Starch-Based Composites for Food Packaging. Polymers 2023, 15, 2972. https://doi.org/10.3390/polym15132972
Muñoz-Gimena PF, Oliver-Cuenca V, Peponi L, López D. A Review on Reinforcements and Additives in Starch-Based Composites for Food Packaging. Polymers. 2023; 15(13):2972. https://doi.org/10.3390/polym15132972
Chicago/Turabian StyleMuñoz-Gimena, Pedro Francisco, Víctor Oliver-Cuenca, Laura Peponi, and Daniel López. 2023. "A Review on Reinforcements and Additives in Starch-Based Composites for Food Packaging" Polymers 15, no. 13: 2972. https://doi.org/10.3390/polym15132972
APA StyleMuñoz-Gimena, P. F., Oliver-Cuenca, V., Peponi, L., & López, D. (2023). A Review on Reinforcements and Additives in Starch-Based Composites for Food Packaging. Polymers, 15(13), 2972. https://doi.org/10.3390/polym15132972