Optimization of Filler Compositions of Electrically Conductive Polypropylene Composites for the Manufacturing of Bipolar Plates
Abstract
:1. Introduction
2. Experimental
2.1. Materials
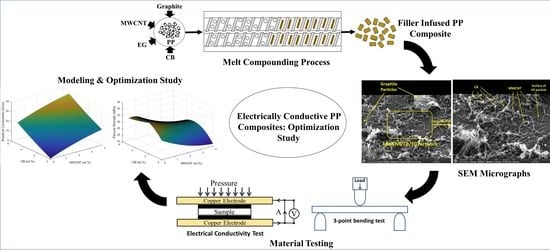
2.2. Experimental Set-Up
3. Characterization
3.1. Through-Plane Electrical Conductivity (TPEC)
3.2. Flexural Strength Testing
3.3. Scanning Electron Microscopy (SEM)
3.4. Design of Experiments
4. Results and Discussion
4.1. Effects of Filler Interaction on the Electrical Conductivity
4.2. Effects of Filler Interaction on the Flexural Strength
4.3. Analysis of Variance
4.4. Factorial Design Analysis
4.4.1. Electrical Conductivity
4.4.2. Flexural Strength
4.5. Response Surface Methodology
4.6. Comparison of Experimental Results with the Commercially Available Materials
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larminie, J.; Dicks, A.; McDonald, M.S. Fuel Cell Systems Explained; J. Wiley: Chichester, UK, 2003. [Google Scholar] [CrossRef]
- Daud, W.; Rosli, R.; Majlan, E.; Hamid, S.; Mohamed, R.; Husaini, T. PEM fuel cell system control: A review. Renew. Energy 2017, 113, 620–638. [Google Scholar] [CrossRef]
- Hermann, A.; Chaudhuri, T.; Spagnol, P. Bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2005, 30, 1297–1302. [Google Scholar] [CrossRef]
- Wilberforce, T.; Ijaodola, O.; Ogungbemi, E.; El Hassan, Z.; Thompson, J.; Olabi, A.G. Effect of bipolar plate materials on performance of fuel cells. In Reference Module in Materials Science and Materials Engineering; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 1–15. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, B.D.; Huang, J.; Baird, D.G. Review of materials and processing methods used in the production of bipolar plates for fuel cells. Int. Mater. Rev. 2007, 52, 1–13. [Google Scholar] [CrossRef]
- Yeetsorn, R.; Fowler, M.W.; Tzoganakis, C. A review of thermoplastic composites for bipolar plate materials in PEM fuel cells. In Nanocomposites with Unique Properties and Applications in Medicine and Industry; InTech: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef] [Green Version]
- Karanfil, G. Importance and applications of DOE/optimization methods in PEM fuel cells: A review. Int. J. Energy Res. 2020, 44, 4–25. [Google Scholar] [CrossRef]
- San, F.G.B.; Tekin, G. A review of thermoplastic composites for bipolar plate applications. Int. J. Energy Res. 2013, 37, 283–309. [Google Scholar] [CrossRef]
- Kahraman, H.; Orhan, M.F. Flow field bipolar plates in a proton exchange membrane fuel cell: Analysis & modeling. Energy Convers. Manag. 2017, 133, 363–384. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Kobayashi, O. Mass production cost of PEM fuel cell by learning curve. Int. J. Hydrogen Energy 2004, 29, 985–990. [Google Scholar] [CrossRef]
- Mighri, F.; Huneault, M.A.; Champagne, M.F. Electrically conductive thermoplastic blends for injection and compression molding of bipolar plates in the fuel cell application. Polym. Eng. Sci. 2004, 44, 1755–1765. [Google Scholar] [CrossRef]
- Utkarsh; Hegab, H.; Tariq, M.; Syed, N.A.; Rizvi, G.; Pop-Iliev, R. Towards Analysis and Optimization of Electrospun PVP (Polyvinylpyrrolidone) Nanofibers. Adv. Polym. Technol. 2020, 2020, 4090747. [Google Scholar] [CrossRef]
- Utkarsh; Syed, N.A.; Tariq, M.; Mohany, A.; Pop-Iliev, R.; Rizvi, G. Experimental investigation of low-frequency sound absorption characteristics of electro-spun Polyvinylpyrrolidone (PVP) membranes. Polymer 2022, 245, 124704. [Google Scholar] [CrossRef]
- Chang, L.-T.; Yu, T.-H.; Huang, H.-H.; Su, Y.-Y.; Tseng, C.-C.; Tsai, H.-J.; Hsu, W.-K. Electrolyte adsorption improved thermoelectric power of non-conductive polymer/carbon nanotubes composites. J. Power Sources 2020, 450, 227651. [Google Scholar] [CrossRef]
- King, J.A.; Johnson, B.A.; Via, M.D.; Ciarkowski, C.J. Electrical conductivity of carbon-filled polypropylene-based resins. J. Appl. Polym. Sci. 2009, 112, 425–433. [Google Scholar] [CrossRef]
- Chen, C.-K.; Kuo, J.-K. Nylon 6/CB polymeric conductive plastic bipolar plates for PEM fuel cells. J. Appl. Polym. Sci. 2006, 101, 3415–3421. [Google Scholar] [CrossRef]
- Heinzel, A.; Mahlendorf, F.; Niemzig, O.; Kreuz, C. Injection moulded low cost bipolar plates for PEM fuel cells. J. Power Sources 2004, 131, 35–40. [Google Scholar] [CrossRef]
- Koncar, G.J.; Marianowski, L.G. Proton Exchange Membrane Fuel Cell Separator Plate. U.S. Patent US5942347A, 24 August 1999. [Google Scholar]
- Simaafrookhteh, S.; Khorshidian, M.; Momenifar, M. Fabrication of multi-filler thermoset-based composite bipolar plates for PEMFCs applications: Molding defects and properties characterizations. Int. J. Hydrogen Energy 2020, 45, 14119–14132. [Google Scholar] [CrossRef]
- Besmann, T.M.; Burchell, T.D. Bipolar Plate/Diffuser for a Proton Exchange Membrane Fuel Cell. U.S. Patent US6171720B1, 9 January 2001. [Google Scholar]
- Radzuan, N.A.M.; Sulong, A.B.; Husaini, T.; Majlan, E.H.; Rosli, M.I.; Aman, M.F. Fabrication of multi-filler MCF/MWCNT/SG-based bipolar plates. Ceram. Int. 2019, 45, 7413–7418. [Google Scholar] [CrossRef]
- Tariq, M.; Utkarsh; Syed, N.A.; Baten, A.; Behravesh, A.H.; Rizvi, G.; Pop-Iliev, R. Effect of Filler Content on the Electrical Conductivity of Graphite Based Composites. In Proceedings of the SPE ANTEC 2021, Online, 10–21 May 2021; pp. 206–209. [Google Scholar]
- Hu, B.; Chang, F.-L.; Xiang, L.-Y.; He, G.-J.; Cao, X.-W.; Yin, X.-C. High performance polyvinylidene fluoride/graphite/multi-walled carbon nanotubes composite bipolar plate for PEMFC with segregated conductive networks. Int. J. Hydrogen Energy 2021, 46, 25666–25676. [Google Scholar] [CrossRef]
- Rzeczkowski, P.; Krause, B.; Pötschke, P. Characterization of Highly Filled PP/Graphite Composites for Adhesive Joining in Fuel Cell Applications. Polymers 2019, 11, 462. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, B.D.; Baird, D.G. Development of bipolar plates for fuel cells from graphite filled wet-lay material and a compatible thermoplastic laminate skin layer. J. Power Sources 2007, 168, 418–425. [Google Scholar] [CrossRef]
- Alo, O.A.; Otunniyi, I.O.; Pienaar, H.; Sadiku, E.R. Electrical and mechanical properties of polypropylene/epoxy blend-graphite/carbon black composite for proton exchange membrane fuel cell bipolar plate. Mater. Today Proc. 2021, 38, 658–662. [Google Scholar] [CrossRef]
- Lawrance, R.J. Low Cost Bipolar Current Collector-Separator for Electrochemical Cells. U.S. Patent US4214969A, 29 July 1980. [Google Scholar]
- Ramírez-Herrera, C.; Tellez-Cruz, M.; Pérez-González, J.; Solorza-Feria, O.; Flores-Vela, A.; Cabañas-Moreno, J. Enhanced mechanical properties and corrosion behavior of polypropylene/multi-walled carbon nanotubes/carbon nanofibers nanocomposites for application in bipolar plates of proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2021, 46, 26110–26125. [Google Scholar] [CrossRef]
- Yan, X.; Liu, J.; Khan, M.A.; Sheriff, S.; Vupputuri, S.; Das, R.; Sun, L.; Young, D.P.; Guo, Z. Efficient solvent-free microwave irradiation synthesis of highly conductive polypropylene nanocomposites with lowly loaded carbon nanotubes. ES Mater. Manuf. 2020, 9, 21–33. [Google Scholar] [CrossRef]
- Dhakate, S.R.; Sharma, S.; Borah, M.; Mathur, R.B.; Dhami, T.L. Expanded graphite-based electrically conductive composites as bipolar plate for PEM fuel cell. Int. J. Hydrogen Energy 2008, 33, 7146–7152. [Google Scholar] [CrossRef]
- Adloo, A.; Sadeghi, M.; Masoomi, M.; Pazhooh, H.N. High performance polymeric bipolar plate based on polypropylene/graphite/graphene/nano-carbon black composites for PEM fuel cells. Renew. Energy 2016, 99, 867–874. [Google Scholar] [CrossRef]
- Liao, W.; Jiang, F.; Zhang, Y.; Zhou, X.; He, Z. Highly-conductive composite bipolar plate based on ternary carbon materials and its performance in redox flow batteries. Renew. Energy 2020, 152, 1310–1316. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lee, Y.-B.; Kim, K.-M.; Jeong, M.-G.; Lim, D.-S. Electrically conductive polymer composite coating on aluminum for PEM fuel cells bipolar plate. Renew. Energy 2013, 54, 46–50. [Google Scholar] [CrossRef]
- Selamat, M.Z.; Sahari, J.; Muhamad, N.; Muchtar, A. Simultaneous Optimization for Multiple Responses on the Compression Moulding Parameters of Composite Graphite—Polypropylene Using Taguchi Method. Key Eng. Mater. 2011, 471–472, 361–366. [Google Scholar] [CrossRef]
- Roncaglia, F.; Romagnoli, M.; Incudini, S.; Santini, E.; Imperato, M.; Spinelli, L.; di Bona, A.; Biagi, R.; Mucci, A. Graphite-epoxy composites for fuel-cell bipolar plates: Wet vs dry mixing and role of the design of experiment in the optimization of molding parameters. Int. J. Hydrogen Energy 2021, 46, 4407–4416. [Google Scholar] [CrossRef]
- San, F.G.B.; Isik-Gulsac, I.; Okur, O. Analysis of the polymer composite bipolar plate properties on the performance of PEMFC (polymer electrolyte membrane fuel cells) by RSM (response surface methodology). Energy 2013, 55, 1067–1075. [Google Scholar] [CrossRef]
- Tariq, M.; Utkarsh; Syed, N.A.; Behravesh, A.H.; Pop-Iliev, R.; Rizvi, G. Synergistic enrichment of electrically conductive polypropylene-graphite composites for fuel cell bipolar plates. Int. J. Energy Res. 2022, 46, 10955–10964. [Google Scholar] [CrossRef]
- Testing protocol for composite bipolar plates. Fuel Cells Bull. 2003, 2003, 5. [CrossRef]
- Pötschke, P.; Bhattacharyya, A.R.; Janke, A.; Goering, H. Melt mixing of polycarbonate/multi-wall carbon nanotube composites. Compos. Interfaces 2003, 10, 389–404. [Google Scholar] [CrossRef]
- Jia, L.-C.; Yan, D.-X.; Cui, C.-H.; Jiang, X.; Ji, X.; Li, Z.-M. Electrically conductive and electromagnetic interference shielding of polyethylene composites with devisable carbon nanotube networks. J. Mater. Chem. C 2015, 3, 9369–9378. [Google Scholar] [CrossRef]
- Kang, K.; Park, S.; Cho, S.-O.; Choi, K.; Ju, H. Development of Lightweight 200-W Direct Methanol Fuel Cell System for Unmanned Aerial Vehicle Applications and Flight Demonstration. Fuel Cells 2014, 14, 694–700. [Google Scholar] [CrossRef]
- Heiser, J.A.; King, J.A.; Konell, J.P.; Sutter, L.L. Electrical conductivity of carbon filled nylon 6,6. Adv. Polym. Technol. 2004, 23, 135–146. [Google Scholar] [CrossRef]
- Krause, B.; Pötschke, P.; Hickmann, T. Improvement of electrical resistivity of highly filled graphite/PP composite based bipolar plates for fuel cells by addition of carbon black. AIP Conf. Proc. 2019, 2139, 110006. [Google Scholar] [CrossRef]
- Wu, K.; Xue, Y.; Yang, W.; Chai, S.; Chen, F.; Fu, Q. Largely enhanced thermal and electrical conductivity via constructing double percolated filler network in polypropylene/expanded graphite–Multi-wall carbon nanotubes ternary composites. Compos. Sci. Technol. 2016, 130, 28–35. [Google Scholar] [CrossRef]
- Sever, K.; Tavman, I.H.; Seki, Y.; Turgut, A.; Omastova, M.; Ozdemir, I. Electrical and mechanical properties of expanded graphite/high density polyethylene nanocomposites. Compos. Part B Eng. 2013, 53, 226–233. [Google Scholar] [CrossRef]
- Yasmin, A.; Luo, J.-J.; Daniel, I.M. Processing of expanded graphite reinforced polymer nanocomposites. Compos. Sci. Technol. 2006, 66, 1182–1189. [Google Scholar] [CrossRef]
- Debelak, B.; Lafdi, K. Use of exfoliated graphite filler to enhance polymer physical properties. Carbon 2007, 45, 1727–1734. [Google Scholar] [CrossRef]
- Khan, W. Carbon Nanotube-Based Polymer Composites: Synthesis, Properties and Applications. In Carbon Nanotubes; Sharma, R., Ed.; IntechOpen: Rijeka, Croatia, 2016; Chapter 1. [Google Scholar] [CrossRef] [Green Version]
- Celzard, A.; Marêché, J.F.; Furdin, G.; Puricelli, S. Electrical conductivity of anisotropic expanded graphite-based monoliths. J. Phys. D Appl. Phys. 2000, 33, 3094–3101. [Google Scholar] [CrossRef]
- Haggenmueller, R.; Gommans, H.; Rinzler, A.; Fischer, J.; Winey, K. Aligned single-wall carbon nanotubes in composites by melt processing methods. Chem. Phys. Lett. 2000, 330, 219–225. [Google Scholar] [CrossRef]
- Bhattacharyya, A.R.; Sreekumar, T.; Liu, T.; Kumar, S.; Ericson, L.M.; Hauge, R.H.; Smalley, R.E. Crystallization and orientation studies in polypropylene/single wall carbon nanotube composite. Polymer 2003, 44, 2373–2377. [Google Scholar] [CrossRef]
- Dhakate, S.; Sharma, S.; Chauhan, N.; Seth, R.; Mathur, R. CNTs nanostructuring effect on the properties of graphite composite bipolar plate. Int. J. Hydrogen Energy 2010, 35, 4195–4200. [Google Scholar] [CrossRef] [Green Version]







| Manufacturers/Patents | Binding Matrix | Graphite Content (wt.%) | Electrical Conductivity (S/cm) | Flexural Strength (MPa) | |
|---|---|---|---|---|---|
| In-Plane | Through-Plane | ||||
| DuPont [5] | - | - | - | 25–33 | 53 |
| GTI (US) [18] | Phenolic | 77.5 | 53 | - | - |
| BMC940-15252A [19] | Vinyl Ester | - | 133 | 25 | 56 |
| SGL [5] | - | - | 100 | 20 | 40 |
| Plug Power [5] | Vinyl Ester | 68 | 55 | - | 40 |
| ORNL (US) [20] | Phenolic | Carbon Fiber | 200 | - | - |
| Control Factors | Symbol | Level 1 | Level 2 | Level 3 |
|---|---|---|---|---|
| MWCNT (wt.%) | A | 0 | 2 | 4 |
| EG (wt.%) | B | 0 | 15 | 30 |
| CB (wt.%) | C | 0 | 2.5 | 5 |
| Run# | A | B | C | Electrical Conductivity (S/cm) (Through-Plane) | Flexural Strength (MPa) |
|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 2.8 ± 0.2 | 30.3 ± 1.3 |
| 2 | 0 | 0 | 2.5 | 9.7 ± 0.4 | 36.9 ± 1.2 |
| 3 | 0 | 0 | 5 | 19.3 ± 1.2 | 34.2 ± 2.8 |
| 4 | 0 | 15 | 0 | 3.6 ± 0.1 | 28.8 ± 0.4 |
| 5 | 0 | 15 | 2.5 | 9.5 ± 0.2 | 31.7 ± 2.3 |
| 6 | 0 | 15 | 5 | 19.3 ± 1.1 | 34.1 ± 2.3 |
| 7 | 0 | 30 | 0 | 4.6 ± 0.1 | 28.7 ± 1.4 |
| 8 | 0 | 30 | 2.5 | 12.2 ± 0.5 | 35.0 ± 2.0 |
| 9 | 0 | 30 | 5 | 24.6 ± 1.5 | 32.2 ± 2.8 |
| 10 | 2 | 0 | 0 | 8.9 ± 0.2 | 28.5 ± 2.1 |
| 11 | 2 | 0 | 2.5 | 19.7 ± 0.5 | 26.1 ± 3.1 |
| 12 | 2 | 0 | 5 | 29.6 ± 0.7 | 25.1 ± 0.9 |
| 13 | 2 | 15 | 0 | 9.5 ± 0.2 | 27.4 ± 2.6 |
| 14 | 2 | 15 | 2.5 | 15.7 ± 0.4 | 34.2 ± 3.8 |
| 15 | 2 | 15 | 5 | 38.8 ± 0.9 | 29.4 ± 1.8 |
| 16 | 2 | 30 | 0 | 10.6 ± 0.3 | 26.9 ± 4.4 |
| 17 | 2 | 30 | 2.5 | 22.3 ± 1.1 | 34.2 ± 1.9 |
| 18 | 2 | 30 | 5 | 32.4 ± 0.3 | 27.4 ± 0.6 |
| 19 | 4 | 0 | 0 | 20.5 ± 1.3 | 23.2 ± 1.9 |
| 20 | 4 | 0 | 2.5 | 31.9 ± 0.8 | 26.5 ± 2.0 |
| 21 | 4 | 0 | 5 | 36.6 ± 0.6 | 28.6 ± 3.2 |
| 22 | 4 | 15 | 0 | 14.0 ± 0.2 | 23.6 ± 1.8 |
| 23 | 4 | 15 | 2.5 | 25.8 ± 0.6 | 26.4 ± 2.9 |
| 24 | 4 | 15 | 5 | 36.4 ± 0.2 | 26.2 ± 2.6 |
| 25 | 4 | 30 | 0 | 12.1 ± 0.6 | 29.1 ± 2.7 |
| 26 | 4 | 30 | 2.5 | 27.5 ± 0.4 | 32.0 ± 3.8 |
| 27 | 4 | 30 | 5 | 39.6 ± 1.1 | 29.4 ± 1.7 |
| Analysis of Variance (ANOVA) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Electrical Conductivity | A1 | 105.6 | B1 | 179 | C1 | 86.6 | (SS)Total | 3304.939 |
| A2 | 187.5 | B2 | 172.6 | C2 | 174.3 | |||
| A3 | 244.4 | B3 | 185.9 | C3 | 276.6 | |||
| SSA | 1081.876 | SSB | 9.831852 | SSC | 2009.503 | (SS)Error | 203.7274 | |
| VA | 540.9381 | VB | 4.915926 | VC | 1004.751 | (V)Error | 10.18637 | |
| (F)A | 53.10411 | (F)B | 0.482598 | (F)C | 98.63685 | |||
| (p)A | 0.0001 | (p)B | 0.6242 | (p)C | 0.0001 | |||
| Flexural Strength | A1 | 291.9 | B1 | 259.4 | C1 | 246.54 | (SS)Total | 344.8688 |
| A2 | 259.24 | B2 | 261.8 | C2 | 283 | |||
| A3 | 245 | B3 | 274.94 | C3 | 266.6 | |||
| SSA | 128.4838 | SSB | 15.55227 | SSC | 74.09982 | (SS)Error | 126.7329 | |
| VA | 64.24191 | VB | 7.776133 | VC | 37.04991 | (V)Error | 6.336644 | |
| (F)A | 10.13816 | (F)B | 1.227169 | (F)C | 5.846929 | |||
| (p)A | 0.0009 | (p)B | 0.3143 | (p)C | 0.01 | |||
| Significant @ 90% confidence level | 2.59 | |||||||
| Significant @ 95% confidence level | 3.49 | |||||||
| Significant @ 99% confidence level | 5.85 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tariq, M.; Utkarsh; Syed, N.A.; Behravesh, A.H.; Pop-Iliev, R.; Rizvi, G. Optimization of Filler Compositions of Electrically Conductive Polypropylene Composites for the Manufacturing of Bipolar Plates. Polymers 2023, 15, 3076. https://doi.org/10.3390/polym15143076
Tariq M, Utkarsh, Syed NA, Behravesh AH, Pop-Iliev R, Rizvi G. Optimization of Filler Compositions of Electrically Conductive Polypropylene Composites for the Manufacturing of Bipolar Plates. Polymers. 2023; 15(14):3076. https://doi.org/10.3390/polym15143076
Chicago/Turabian StyleTariq, Muhammad, Utkarsh, Nabeel Ahmed Syed, Amir Hossein Behravesh, Remon Pop-Iliev, and Ghaus Rizvi. 2023. "Optimization of Filler Compositions of Electrically Conductive Polypropylene Composites for the Manufacturing of Bipolar Plates" Polymers 15, no. 14: 3076. https://doi.org/10.3390/polym15143076
APA StyleTariq, M., Utkarsh, Syed, N. A., Behravesh, A. H., Pop-Iliev, R., & Rizvi, G. (2023). Optimization of Filler Compositions of Electrically Conductive Polypropylene Composites for the Manufacturing of Bipolar Plates. Polymers, 15(14), 3076. https://doi.org/10.3390/polym15143076