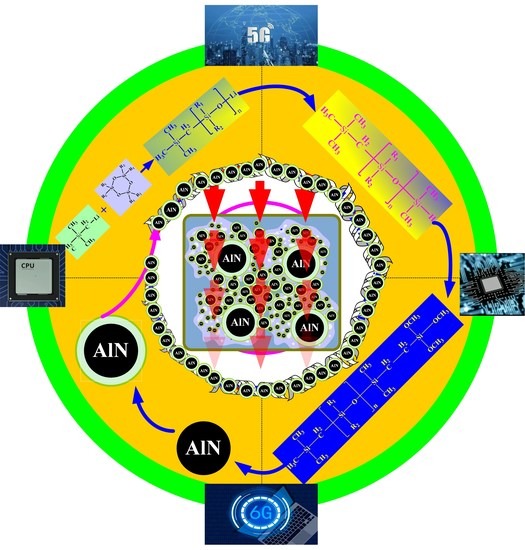
Preparation of Monotrimethoxylsilylethyl-Terminated Polysiloxane Fluids and Their Application in Thermal Interface Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Materials
2.1.1. α-Trimethylsilylmethyl-ω-Trimethoxylsilylethyl-Terminated Polydimethylsiloxane (TMSM-PDMS-TMOS)
TMSM-PDMS-DMS
TMSM-PDMS-TMOS
2.1.2. α-Trimethylsilylmethyl-ω-Trimethoxylsilylethyl-Terminated Poly[2,2,2-Trifluoropropyl(methyl)siloxane] (TMSM-PTFPMS-TMOS)
TMSM-PTFPMS-DMS
TMSM-PTFPMS-TMOS
2.1.3. α-Trimethylsilylmethyl-ω-Trimethoxylsilylethyl-Terminated Polydiethylsiloxane (TMSM-PDES-TMOS)
TMSM-PDES-DMS
TMSM-PDES-TMOS
2.2. Charactrization
2.3. Preparation of Thermally Conductive Composites
2.3.1. Thermally Conductive Paste
2.3.2. Thermal Silicone Rubber
3. Results and Discussion
3.1. Effect of Reaction Time on the Yield of TMSM-PDMS-DMS
3.2. Effect of Solvent Types on the Yield of TMSM-PTFPMS-DMS
3.3. Effect of Water Content and Monomer Structure on Yield of Polymer
3.4. Effect of Polysiloxane Structure on Thermal Conductivity of AlN-Filled Composites
3.4.1. TMSM-PDMS-TMOS
3.4.2. TMSM-PTFPMS-TMOS
3.4.3. TMSM-PDES-TMOS
3.5. Effect of Polysiloxane Structure on the Surface and Interface Morphology of Composite Materials
3.6. Possible Mechanism of Surface Treatment of AlN Fillers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahil, K.M.F.; Balandin, A.A. Thermal properties of graphene and multilayer graphene: Applications in thermal interface materials. Solid State Commun. 2012, 152, 1331–1340. [Google Scholar] [CrossRef]
- Otiaba, K.C.; Ekere, N.N.; Bhatti, R.S.; Mallik, S.; Alam, M.O.; Amalu, E.H. Thermal interface materials for automotive electronic control unit: Trends, technology and R&D challenges. Microelectron. Reliab. 2011, 51, 2031–2043. [Google Scholar]
- Sevinchan, E.; Dincer, I.; Lang, H. A review on thermal management methods for robots. Appl. Therm. Eng. 2018, 140, 799–813. [Google Scholar] [CrossRef]
- Zhang, Y.; Heo, Y.J.; Son, Y.R.; In, I.; An, K.H.; Kim, B.J.; Park, S.J. Recent advanced thermal interfacial materials: A review of conducting mechanisms and parameters of carbon materials. Carbon 2019, 142, 445–460. [Google Scholar] [CrossRef]
- Zhao, J.W.; Zhao, R.; Huo, Y.K.; Cheng, W.L. Effects of surface roughness, temperature and pressure on interface thermal resistance of thermal interface materials. Int. J. Heat Mass Transf. 2019, 140, 705–716. [Google Scholar] [CrossRef]
- Lule, Z.; Kim, J. Thermally conductive and highly rigid polylactic acid (PLA) hybrid composite filled with surface treated alumina/nano-sized aluminum nitride. Compos. Part A Appl. Sci. Manuf. 2019, 124, 105506. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Sun, B.; Jiang, P. Highly thermally conductive yet electrically insulating polymer/boron nitride nanosheets nanocomposite films for improved thermal management capability. ACS Nano 2019, 13, 337–345. [Google Scholar] [CrossRef]
- Dai, W.; Lv, L.; Lu, J.; Hou, H.; Yan, Q.; Alam, F.E.; Li, Y.; Zeng, X.; Yu, J.; Wei, Q.; et al. A paper-like inorganic thermal interface material composed of hierarchically structured graphene/silicon carbide nanorods. ACS Nano 2019, 13, 1547–1554. [Google Scholar] [CrossRef]
- Xu, X.; Chen, J.; Zhou, J.; Li, B. Thermal conductivity of polymers and their nanocomposites. Adv. Mater. 2018, 30, 1705544. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Wang, X.; Zhou, J.; Song, B.; Jiang, Z.; Lee, E.M.; Huberman, S.; Gleason, K.K.; Chen, G. Molecular engineered conjugated polymer with high thermal conductivity. Sci. Adv. 2018, 4, 2375–2548. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.W.; Zheng, S.; Kim, M.S.; Cheon, S.A.; Ko, S.; Park, T.J. Development of specific immobilization method on gold surface and its application for determining cardiac risk. BioChip J. 2014, 8, 295–302. [Google Scholar] [CrossRef]
- Shen, Z.; Feng, J. Highly thermally conductive composite films based on nanofibrillated cellulose in situ coated with a small amount of silver nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 24193–24200. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Liu, J.; Liu, B.; Wu, J.; Cheng, H.M.; Kang, F. Two-Dimensional Materials for Thermal Management Applications. Joule 2018, 2, 442–463. [Google Scholar] [CrossRef] [Green Version]
- Hou, H.; Dai, W.; Yan, Q.; Lv, L.; Alam, F.E.; Yang, M.; Yao, Y.; Zeng, X.; Xu, J.B.; Yu, J.; et al. Graphene size-dependent modulation of graphene frameworks contributing to the superior thermal conductivity of epoxy composites. J. Mater. Chem. A 2018, 6, 12091–12097. [Google Scholar] [CrossRef]
- Xue, Y.; Zhou, X.; Zhan, T.; Jiang, B.; Guo, Q.; Fu, X.; Shimamura, K.; Xu, Y.; Mori, T.; Dai, P.; et al. Densely interconnected porous BN frameworks for multifunctional and isotropicallythermoconductive polymer composites. Adv. Funct. Mater. 2018, 28, 1801205. [Google Scholar] [CrossRef] [Green Version]
- Molenberg, A.; Möller, M. Structure and phase transitions of poly (diethylsiloxane). Macromolecules 1997, 30, 8332–8337. [Google Scholar] [CrossRef]
- Zhang, K.; Cai, J.; Li, X.; Li, H.; Zhao, Y.H.; Yuan, X.Y. Balance of polyacrylate-fluorosilicone block copolymers as icephobic coatings. Chin. J. Polym. Sci. 2015, 33, 153–162. [Google Scholar] [CrossRef]
- Obrezkova, M.A.; Selezneva, E.V.; Demchenko, N.V.; Möller, M.; Kotov, V.M. Polydiethylsiloxane macroinitiators for the synthesis of block copolymers. J. Nesmeyanov Inst. Organoelement Compd. Russ. Acad. Sci. 2020, 3, 176–181. [Google Scholar] [CrossRef]
- Tsuchihara, K.; Fujishige, S. Living polymerization of hexaethylcyclotrisiloxane. Polym. Bull. 1999, 43, 129–134. [Google Scholar] [CrossRef]
- Out, G.J.J.; Turetskii, A.A.; Snijder, M.; Möller, M.; Papkov, V.S. Model polydiethylsiloxane networks: 1. Synthesis and phase behavior. Polymer 1995, 36, 3213–3221. [Google Scholar] [CrossRef] [Green Version]
- Hedden, R.C.; Cohen, C. Preparation of poly (diethylsiloxane) with the NaOH/12-crown-4 catalyst. Polymer 2000, 41, 6975–6979. [Google Scholar] [CrossRef]














| Sample ID | Mn (g/mol) | Mw (g/mol) | PDI | M b NMR (g/mol) |
|---|---|---|---|---|
| TMSM-PDMS-DMS | 1611 | 2048 | 1.27 | 908 |
| TMSM-PDMS-TMOS | ND a | ND | ND | 1056 |
| TMSM-PTFPMS-DMS | 857 | 1051 | 1.23 | 1628 |
| TMSM-PTFPMS-TMOS | ND | ND | ND | 1776 |
| TMSM-PDES-DMS | 718 | 879 | 1.22 | 782 |
| TMSM-PDES-TMOS | ND | ND | ND | 930 |
| Entry | n* | mD3/g | VTMSiMLi/mL | VTHF/mL | mDMCS/g | t/h | T/°C | Yield/% |
|---|---|---|---|---|---|---|---|---|
| A1 | 10 | 10.00 | 20.00 | 30.00 | 1.75 | 15 | 40 | 59.0 |
| A2 | 10 | 10.06 | 20.00 | 30.00 | 1.75 | 13 | 40 | 50.1 |
| A3 | 10 | 10.07 | 20.00 | 30.00 | 1.75 | 9 | 40 | 60.1 |
| A4 | 10 | 10.00 | 20.00 | 30.00 | 1.75 | 6 | 40 | 80.0 |
| Entry | n* | mD3F/g | VTMSiMLi/mL | V1/mL | mDMCS/g | Solvent | t/h | T/°C | Yield/% |
|---|---|---|---|---|---|---|---|---|---|
| B1 | 10 | 10.02 | 12.00 | 10.00 | 1.25 | THF | 8 | 40 | 50.1 |
| B2 | 10 | 10.02 | 12.00 | 10.00 | 1.25 | DMSO | 8 | 40 | 18.0 |
| B3 | 10 | 10.02 | 15.00 | 10.00 | 1.25 | NMP | 8 | 40 | 42.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Long, X.; Wang, Y.; Wu, C.; Qu, Z.; Pei, Z.; Shi, C.; Wang, T.; Dong, H. Preparation of Monotrimethoxylsilylethyl-Terminated Polysiloxane Fluids and Their Application in Thermal Interface Materials. Polymers 2023, 15, 3334. https://doi.org/10.3390/polym15163334
Liu Y, Long X, Wang Y, Wu C, Qu Z, Pei Z, Shi C, Wang T, Dong H. Preparation of Monotrimethoxylsilylethyl-Terminated Polysiloxane Fluids and Their Application in Thermal Interface Materials. Polymers. 2023; 15(16):3334. https://doi.org/10.3390/polym15163334
Chicago/Turabian StyleLiu, Yang, Xu Long, Yang Wang, Chuan Wu, Zhirong Qu, Zhiwei Pei, Chunlong Shi, Ting Wang, and Hong Dong. 2023. "Preparation of Monotrimethoxylsilylethyl-Terminated Polysiloxane Fluids and Their Application in Thermal Interface Materials" Polymers 15, no. 16: 3334. https://doi.org/10.3390/polym15163334
APA StyleLiu, Y., Long, X., Wang, Y., Wu, C., Qu, Z., Pei, Z., Shi, C., Wang, T., & Dong, H. (2023). Preparation of Monotrimethoxylsilylethyl-Terminated Polysiloxane Fluids and Their Application in Thermal Interface Materials. Polymers, 15(16), 3334. https://doi.org/10.3390/polym15163334