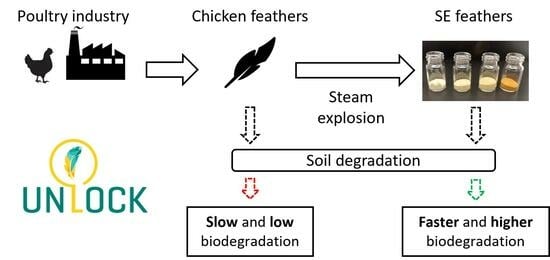
Enhanced Biodegradability in Soil of Chicken Feather by Steam Explosion for Potential Application in Agricultural Biodegradable Plastics
Abstract
:1. Introduction
2. Experimental
2.1. Obtention and Conditioning of Chicken Feathers
2.2. Steam Explosion and Processing of Chicken Feathers
2.3. Characterization of Chicken Feathers
2.3.1. Density of CF
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.3. Thermogravimetry (TGA)
2.3.4. Field Emission Scanning Electron Microscopy (FE-SEM)
2.3.5. X-ray Diffraction (XRD)
2.3.6. Elemental Analysis
2.3.7. Biodegradation in Soil
3. Results and Discussion
3.1. Effect of SE on CF Density and Yield
3.2. Chemical Structure of SE Feathers
3.3. Elemental Analysis of SE Feathers
3.4. Thermal Stability of SE Feathers
3.5. Morphology of SE Feathers
3.6. X-ray Diffraction of the SE Feathers
3.7. Biodegradation in Soil of SE Feathers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Grazziotin, A.; Pimentel, F.A.; De Jong, E.V.; Brandelli, A. Nutritional Improvement of Feather Protein by Treatment with Microbial Keratinase. Anim. Feed. Sci. Technol. 2006, 126, 135–144. [Google Scholar] [CrossRef]
- Sinkiewicz, I.; Śliwińska, A.; Staroszczyk, H.; Kołodziejska, I. Alternative Methods of Preparation of Soluble Keratin from Chicken Feathers. Waste Biomass Valorization 2017, 8, 1043–1048. [Google Scholar] [CrossRef]
- Suzuki, Y.; Tsujimoto, Y.; Matsui, H.; Watanabe, K. Decomposition of Extremely Hard-to-Degrade Animal Proteins by Thermophilic Bacteria. J. Biosci. Bioeng. 2006, 102, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Gupta, A. Sustainable Management of Keratin Waste Biomass: Applications and Future Perspectives. Braz. Arch. Biol. Technol. 2016, 59, e16150684. [Google Scholar] [CrossRef]
- Taskin, M.; Kurbanoglu, E.B. Evaluation of Waste Chicken Feathers as Peptone Source for Bacterial Growth. J. Appl. Microbiol. 2011, 111, 826–834. [Google Scholar] [CrossRef]
- Du, W.; Zhang, L.; Zhang, C.; Cao, J.; Wang, D.; Li, H.; Li, W.; Zeng, J. Green and Highly Efficient Wool Keratin Extraction by Microwave Induction Method. Front. Mater. 2022, 8, 789081. [Google Scholar] [CrossRef]
- McKittrick, J.; Chen, P.-Y.; Bodde, S.G.; Yang, W.; Novitskaya, E.E.; Meyers, M.A. The Structure, Functions, and Mechanical Properties of Keratin. JOM 2012, 64, 449–468. [Google Scholar] [CrossRef]
- Zoccola, M.; Aluigi, A.; Tonin, C. Characterisation of Keratin Biomass from Butchery and Wool Industry Wastes. J. Mol. Struct. 2009, 938, 35–40. [Google Scholar] [CrossRef]
- Schmidt, W.F.; Jayasundera, S. Microcrystalline Avian Keratin Protein Fibers. In Natural Fibers, Plastics and Composites; Springer: New York, NY, USA, 2004. [Google Scholar]
- Feughelman, M. Natural Protein Fibers. J. Appl. Polym. Sci. 2002, 83, 489–507. [Google Scholar] [CrossRef]
- Guilherme, G.E.; Burin, G.R.M.; de Muniz, G.I.B.; Alves, H.J. Valorization of Feather Waste in Brazil: Structure, Methods of Extraction, and Applications of Feather Keratin. Environ. Sci. Pollut. Res. 2023, 30, 39558–39567. [Google Scholar]
- El Boushy, A.R.; Van der Poel, A.F.; Poel, A.F.B. Handbook of Poultry Feed from Waste: Processing and Use; Springer: Dordrecht, The Netherlands, 2000; ISBN 978-94-017-1750-2. [Google Scholar]
- Holkar, C.R.; Jadhav, A.J.; Bhavsar, P.S.; Kannan, S.; Pinjari, D.V.; Pandit, A.B. Acoustic Cavitation Assisted Alkaline Hydrolysis of Wool Based Keratins to Produce Organic Amendment Fertilizers. ACS Sustain. Chem. Eng. 2016, 4, 2789–2796. [Google Scholar] [CrossRef]
- Moritz, J.S.; Latshaw, J.D. Indicators of Nutritional Value of Hydrolyzed Feather Meal. Poult. Sci. 2001, 80, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Parsons, C.M. Effect of Processing Systems on Protein Quality of Feather Meals and Hog Hair Meals. Poult. Sci. 1997, 76, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; El Boushy, A.R.; Roodbeen, A.E.; Ketelaars, E.H. Effects of Processing Time and Moisture Content on Amino Acid Composition Adn Nitrogen Characteristics of Feather Meal. Anim. Feed. Sci. Technol. 1986, 34, 1986. [Google Scholar]
- George, B.R.; Evazynajad, A.; Bockarie, A.; McBride, H.; Bunik, T.; Scutti, A. Keratin Fiber Nonwovens for Erosion Control. In Natural Fibers, Plastics and Composites; Wallenberger, F.T., Weston, N.E., Eds.; Springer: Boston, MA, USA, 2004; pp. 67–81. ISBN 9781461347743. [Google Scholar]
- Agbeboh, N.I.; Oladele, I.O.; Daramola, O.O.; Adediran, A.A.; Olasukanmi, O.O.; Tanimola, M.O. Environmentally Sustainable Processes for the Synthesis of Hydroxyapatite. Heliyon 2020, 6, e03765. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, P.; Dumonceaux, T.; Tabil, L.G.; Mupondwa, E.; Soleimani, M.; Cree, D. Steam Explosion Pre-Treatment of Sawdust for Biofuel Pellets. Clean. Technol. 2022, 4, 1175–1192. [Google Scholar] [CrossRef]
- Emmel, A.; Mathias, A.L.; Wypych, F.; Ramos, L.P. Fractionation of Eucalyptus Grandis Chips by Dilute Acid-Catalysed Steam Explosion. Bioresour. Technol. 2003, 86, 105–115. [Google Scholar] [CrossRef]
- Fengel, D.; Wegner, G. Wood Chemistry, Ultrastructure, Reactions. In Wood; De Gruyer: Berlin, German, 1983. [Google Scholar]
- Rodríguez, F.; Sanchez, A.; Parra, C. Role of Steam Explosion on Enzymatic Digestibility, Xylan Extraction, and Lignin Release of Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2017, 5, 5234–5240. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, B.; Yu, F.; Xu, G.; Song, A. A Real Explosion: The Requirement of Steam Explosion Pretreatment. Bioresour. Technol. 2012, 121, 335–341. [Google Scholar] [CrossRef]
- Garrote, G.; Domínguez, H.; Parajó, J.C. Hydrothermal Processing of Lignocellulosic Materials. Holz Als Roh-Und Werkst. 1999, 57, 191–202. [Google Scholar] [CrossRef]
- Pielhop, T.; Amgarten, J.; von Rohr, P.R.; Studer, M.H. Steam Explosion Pretreatment of Softwood: The Effect of the Explosive Decompression on Enzymatic Digestibility. Biotechnol. Biofuels 2016, 9, 152. [Google Scholar] [CrossRef]
- Xu, W.; Ke, G.; Wu, J.; Wang, X. Modification of Wool Fiber Using Steam Explosion. Eur. Polym. J. 2006, 42, 2168–2173. [Google Scholar] [CrossRef]
- Tonin, C.; Zoccola, M.; Aluigi, A.; Varesano, A.; Montarsolo, A.; Vineis, C.; Zimbardi, F. Study on the Conversion of Wool Keratin by Steam Explosion. Biomacromolecules 2006, 7, 3499–3504. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.D.; Karunakaran, C.; Tabil, L.G. Physicochemical Characteristics of Densified Untreated and Steam Exploded Poplar Wood and Wheat Straw Grinds. Biosyst. Eng. 2009, 103, 198–207. [Google Scholar] [CrossRef]
- Overen, R.P.; Chornet, E.; Gascoigne, J.A. Fractionation of Lignocellulosics by Steam-Aqueous Pretreatments. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar] [CrossRef]
- ISO 9427; Wood-Based Panels—Determination of Density. ISO: Geneva, Switzerland, 2003.
- Ha, S.W.; Tonelli, A.E.; Hudson, S.M. Structural Studies of Bombyx Mori Silk Fibroin during Regeneration from Solutions and Wet Fiber Spinning. Biomacromolecules 2005, 6, 1722–1731. [Google Scholar] [CrossRef]
- Hernandez, A.L.M.; Santos, C.V.; de Icaza, M.; Castano, V.M. Microstructural Characterisation of Keratin Fibres from Chicken Feathers. Int. J. Environ. Pollut. 2005, 23, 162. [Google Scholar] [CrossRef]
- Senoz, E.; Wool, R.P. Microporous Carbon-Nitrogen Fibers from Keratin Fibers by Pyrolysis. J. Appl. Polym. Sci. 2010, 118, 1752–1765. [Google Scholar] [CrossRef]
- Fu, K.; Griebenow, K.; Hsieh, L.; Klibanov, A.M.; Robert, L. FTIR Characterization of the Secondary Structure of Proteins Encapsulated within PLGA Microspheres. J. Control. Release 1999, 58, 357–366. [Google Scholar] [CrossRef]
- Cai, S.; Singh, B.R. Identification of B-Turn and Random Coil Amide III Infrared Bands for Secondary Structure Estimation of Proteins. Biophys. Chem. 1999, 80, 7–20. [Google Scholar] [CrossRef]
- Khosa, M.A.; Wu, J.; Ullah, A. Chemical Modification, Characterization, and Application of Chicken Feathers as Novel Biosorbents. RSC Adv. 2013, 3, 20800–20810. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, R.; Zhang, Y.; Wu, L. Sustainable and Practical Utilization of Feather Keratin by an Innovative Physicochemical Pretreatment: High Density Steam Flash-Explosion. Green. Chem. 2012, 14, 3352–3360. [Google Scholar] [CrossRef]
- Kumar, A.; Tateyama, S.; Yasaki, K.; Asif Ali, M.; Takaya, N.; Singh, R.; Kaneko, T. Ultrahigh Performance Bio-Based Polyimides from 4,4′-Diaminostilbene. Polymer 2015, 83, 182–189. [Google Scholar] [CrossRef]
- Cao, J.; Billows, C.A. Crystallinity Determination of Native and Stretched Wool by X-Ray Diffraction. Polym. Int. 1999, 48, 1027–1033. [Google Scholar] [CrossRef]
- ISO 17556; Plastics—Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in Soil by Measuring the Oxygen Demand in a Respirometer or the Amount of Carbon Dioxide Evolved. ISO: Geneva, Switzerland, 2019.
- Briassoulis, D.; Mistriotis, A. Key Parameters in Testing Biodegradation of Bio-Based Materials in Soil. Chemosphere 2018, 207, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Lin, L.; Xu, E. Functional Pretreatments of Natural Raw Materials. In Advanced High Strength Natural Fibre Composites in Construction; Elsevier: Amsterdam, The Netherlands, 2017; pp. 87–114. [Google Scholar] [CrossRef]
- Fagbemi, O.D.; Sithole, B.; Tesfaye, T. Optimization of Keratin Protein Extraction from Waste Chicken Feathers Using Hybrid Pre-Treatment Techniques. Sustain. Chem. Pharm. 2020, 17, 100267. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, J.; Ren, X.; Lau, A.; Rezaei, H.; Takada, M.; Bi, X.; Sokhansanj, S. Steam Explosion of Lignocellulosic Biomass for Multiple Advanced Bioenergy Processes: A Review. Renew. Sustain. Energy Rev. 2022, 154, 111871. [Google Scholar] [CrossRef]
- Aluigi, A.; Zoccola, M.; Vineis, C.; Tonin, C.; Ferrero, F.; Canetti, M. Study on the Structure and Properties of Wool Keratin Regenerated from Formic Acid. Int. J. Biol. Macromol. 2007, 41, 266–273. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Natural Fibers, Biopolymers, and Biocomposites, 1st ed.; Mohanty, A.K., Misra, M., Drzal, L.T., Eds.; CRC Press: Boca Raton, FL, USA, 2005; Volume 1, ISBN 9780203508206. [Google Scholar]
- Eslahi, N.; Dadashian, F.; Nejad, N.H. An Investigation on Keratin Extraction from Wool and Feather Waste by Enzymatic Hydrolysis. Prep. Biochem. Biotechnol. 2013, 43, 624–648. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kris, G.S. Introduction to Spectroscopy, 2nd ed.; Saunders Golden Sunburst Series: Orlando, FL, USA, 1979; Volume 56. [Google Scholar]
- Vasconcelos, A.; Freddi, G.; Cavaco-Paulo, A. Biodegradable Materials Based on Silk Fibroin and Keratin. Biomacromolecules 2008, 9, 1299–1305. [Google Scholar] [CrossRef]
- Fraser, R.D.B.; Parry, D.A.D. Molecular Packing in the Feather Keratin Filament. J. Struct. Biol. 2008, 162, 1–13. [Google Scholar] [CrossRef]
- Mattiello, S.; Guzzini, A.; Del Giudice, A.; Santulli, C.; Antonini, M.; Lupidi, G.; Gunnella, R. Physico-Chemical Characterization of Keratin from Wool and Chicken Feathers Extracted Using Refined Chemical Methods. Polymers 2023, 15, 181. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, W.; Yang, R. Steam Flash Explosion Assisted Dissolution of Keratin from Feathers. ACS Sustain. Chem. Eng. 2015, 3, 2036–2042. [Google Scholar] [CrossRef]
- Kambe, Y.; Mizoguchi, Y.; Kuwahara, K.; Nakaoki, T.; Hirano, Y.; Yamaoka, T. Beta-Sheet Content Significantly Correlates with the Biodegradation Time of Silk Fibroin Hydrogels Showing a Wide Range of Compressive Modulus. Polym. Degrad. Stab. 2020, 179, 109240. [Google Scholar] [CrossRef]
- Chen, D.; Pinho, L.S.; Federici, E.; Zuo, X.; Ilavsky, J.; Kuzmenko, I.; Yang, Z.; Jones, O.G.; Campanella, O. Heat Accelerates Degradation of β-Lactoglobulin Fibrils at Neutral PH. Food Hydrocoll. 2022, 124, 107291. [Google Scholar] [CrossRef]
- Nuutinen, M. Feather Characterization and Processing. Master’s Thesis, Aalto University School of Chemical Engineering, Aalto, Finland, 2017. [Google Scholar]
- Wang, Z.; Li, Y.; Jiang, L.; Qi, B.; Zhou, L. Relationship between Secondary Structure and Surface Hydrophobicity of Soybean Protein Isolate Subjected to Heat Treatment. J. Chem. 2014, 2014, 475389. [Google Scholar] [CrossRef]
- Tesfaye, T.; Sithole, B.; Ramjugernath, D.; Chunilall, V. Valorisation of Chicken Feathers: Characterisation of Chemical Properties. Waste Manag. 2017, 68, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Mastalygina, E.E.; Popov, A.A.; Pantyukhov, P. V Effect of Biobased Fillers Nature on Biodeterioration of Hybrid Polyethylene Composites by Mold Fungi. IOP Conf. Ser. Mater. Sci. Eng. 2017, 213, 012011. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, R.; Zhao, W. Improving Digestibility of Feather Meal by Steam Flash Explosion. J. Agric. Food Chem. 2014, 62, 2745–2751. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, F.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Tesfaye, T.; Sithole, B.; Ramjugernath, D.; Chunilall, V. Valorisation of Chicken Feathers: Application in Paper Production. J. Clean. Prod. 2017, 164, 1324–1331. [Google Scholar] [CrossRef]
- Pereira Marques, F.; Lima Soares, A.K.; Lomonaco, D.; Alexandre e Silva, L.M.; Tédde Santaella, S.; de Freitas Rosa, M.; Carrhá Leitão, R. Steam Explosion Pretreatment Improves Acetic Acid Organosolv Delignification of Oil Palm Mesocarp Fibers and Sugarcane Bagasse. Int. J. Biol. Macromol. 2021, 175, 304–312. [Google Scholar] [CrossRef]
- Ma, C.; Ni, L.; Guo, Z.; Zeng, H.; Wu, M.; Zhang, M.; Zheng, B. Principle and Application of Steam Explosion Technology in Modification of Food Fiber. Foods 2022, 11, 3370. [Google Scholar] [CrossRef] [PubMed]
- Renneckar, S.; Zink-Sbarp, A.; Glasser, W.G. Fiber Modification by Steam Explosion: Microscopic Analysis of Co-Refined Wood and Polypropylene. IAWA J. 2007, 28, 13–27. [Google Scholar] [CrossRef]
- Muniyasamy, S.; Muniyasamy, S.; John, M.J.; John, M.J. Biodegradability of Biobased Polymeric Materials in Natural Environments. In Handbook of Composites from Renewable Materials; Wiley: Hoboken, NJ, USA, 2017; pp. 625–653. [Google Scholar]
- Sharma, S.; Gupta, A.; Kumar, A.; Kee, C.G.; Kamyab, H.; Saufi, S.M. An Efficient Conversion of Waste Feather Keratin into Ecofriendly Bioplastic Film. Clean. Technol. Environ. Policy 2018, 20, 2157–2167. [Google Scholar] [CrossRef]
- Cao, J. Is the A-b Transition of Keratin a Transition of a-Helices to b-Pleated Sheets? Part I: In Situ XRD Studies. J. Mol. Struct. 2000, 553, 101–107. [Google Scholar] [CrossRef]
- Sheng, Z.; Gao, J.; Jin, Z.; Dai, H.; Zheng, L.; Wang, B. Effect of Steam Explosion on Degumming Efficiency and Physicochemical Characteristics of Banana Fiber. J. Appl. Polym. Sci. 2014, 131, 40598. [Google Scholar] [CrossRef]
- Zhang, X.; Han, G.; Jiang, W.; Zhang, Y.; Li, X.; Li, M. Effect of Steam Pressure on Chemical and Structural of Kenaf Fibers during Steam Explosion. Bioresources 2016, 11, 6590–6599. [Google Scholar] [CrossRef]
- Jacquet, N.; Vanderghem, C.; Danthine, S.M.; Blecker, C. Influence of Steam Explosion on the Crystallinity of Cellulose Fiber. In Proceedings of the 19th National Symposium on Applied Biological Sciences, Gembloux, Belgium, 7 February 2014. [Google Scholar]






| Samples | Steam Explosion | Grinding | Yield (%) | Bulk Density (g cm−3) | ||
|---|---|---|---|---|---|---|
| Temperature (°C) | Residence Time (min) | Severity Factor | Particle Size (mm) | |||
| Raw feathers-0.5 mm | - | - | - | 0.5 | - | 0.015 ± 0.01 |
| CF-SE-160 °C-2 min-0.5 mm | 160 | 2 | 2.07 | 0.5 | 100 | 0.306 ± 0.02 |
| CF-SE-160 °C-4 min-0.5 mm | 4 | 2.37 | 0.5 | 80 | 0.447 ± 0.02 | |
| CF-SE-180 °C-2 min-0.5 mm | 180 | 2 | 2.66 | 0.5 | 85 | 0.565 ± 0.04 |
| CF-SE-190 °C-4 min-0.5 mm | 190 | 4 | 3.25 | 0.5 | 80 | 0.706 ± 0.03 |
| Samples | α-Helix (%) | β-Sheet (%) | β-Turns (%) | Random Coil (%) |
|---|---|---|---|---|
| Raw feathers-0.5 mm | 13 ± 4 | 72 ± 3 | 10 ± 2 | 3 ± 1 |
| CF-SE-160 °C-2 min-0.5 mm | 18 ± 2 | 63 ± 4 | 3 ± 1 | 14 ± 4 |
| CF-SE-160 °C-4 min-0.5 mm | 20 ± 5 | 62 ± 2 | 5 ± 2 | 14 ± 5 |
| CF-SE-180 °C-2 min-0.5 mm | 28 ± 2 | 53 ± 5 | 3 ± 1 | 17 ± 4 |
| CF-SE-190 °C-4 min-0.5 mm | 29 ± 2 | 52 ± 1 | 4 ± 1 | 16 ± 3 |
| Samples | Severity Factor | Relative Crystallinity (%) |
|---|---|---|
| Raw feathers-0.5mm | - | 0.35 ± 0.01 |
| CF-SE-160°C-2 min-0.5 mm | 2.07 | 0.45 ± 0.01 |
| CF-SE-160°C-4 min-0.5 mm | 2.37 | 0.40 ± 0.02 |
| CF-SE-180°C-2 min-0.5 mm | 2.66 | 0.39 ± 0.01 |
| CF-SE-190°C-4 min-0.5 mm | 3.25 | 0.26 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vadillo, J.; Montes, S.; Grande, H.-J.; Verstichel, S.; Almqvist, J.; Wrześniewska-Tosik, K. Enhanced Biodegradability in Soil of Chicken Feather by Steam Explosion for Potential Application in Agricultural Biodegradable Plastics. Polymers 2023, 15, 3701. https://doi.org/10.3390/polym15183701
Vadillo J, Montes S, Grande H-J, Verstichel S, Almqvist J, Wrześniewska-Tosik K. Enhanced Biodegradability in Soil of Chicken Feather by Steam Explosion for Potential Application in Agricultural Biodegradable Plastics. Polymers. 2023; 15(18):3701. https://doi.org/10.3390/polym15183701
Chicago/Turabian StyleVadillo, Julen, Sarah Montes, Hans-Jürgen Grande, Steven Verstichel, Jonna Almqvist, and Krystyna Wrześniewska-Tosik. 2023. "Enhanced Biodegradability in Soil of Chicken Feather by Steam Explosion for Potential Application in Agricultural Biodegradable Plastics" Polymers 15, no. 18: 3701. https://doi.org/10.3390/polym15183701
APA StyleVadillo, J., Montes, S., Grande, H. -J., Verstichel, S., Almqvist, J., & Wrześniewska-Tosik, K. (2023). Enhanced Biodegradability in Soil of Chicken Feather by Steam Explosion for Potential Application in Agricultural Biodegradable Plastics. Polymers, 15(18), 3701. https://doi.org/10.3390/polym15183701