Sustainable Collagen Composites with Graphene Oxide for Bending Resistive Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Sample Characterization
2.4. Bending Sensor Developed
3. Results and Discussion
3.1. Thermal and Physicochemical Properties
3.2. Morphological and Surface Analyses
3.3. Mechanical Properties
3.4. Electrical Properties
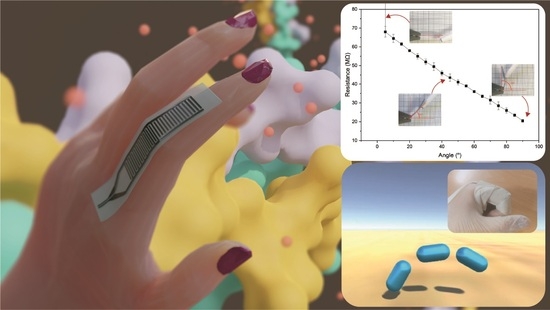
3.5. Functional Bending Sensor Response
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwager, P.; Decker, N.; Kaltenegger, I. Exploring Green Chemistry, Sustainable Chemistry and innovative business models such as Chemical Leasing in the context of international policy discussions. Curr. Opin. Green Sustain. Chem. 2016, 1, 18–21. [Google Scholar] [CrossRef]
- Martínez, J.; Cortés, J.F.; Miranda, R. Green Chemistry Metrics, A Review. Processes 2022, 10, 1274. [Google Scholar] [CrossRef]
- Sajid, M.; Płotka-Wasylka, J. Green analytical chemistry metrics: A review. Talanta 2022, 238, 123046. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Yu, I.K.M.; Tsang, D.C.W.; Bolan, N.S.; Sik Ok, Y.; Igalavithana, A.D.; Kirkham, M.B.; Kim, K.-H.; Vikrant, K. Value-added chemicals from food supply chain wastes: State-of-the-art review and future prospects. Chem. Eng. J. 2019, 375, 121983. [Google Scholar] [CrossRef]
- Silva, A.C.Q.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Natural Polymers-Based Materials: A Contribution to a Greener Future. Molecules 2022, 27, 94. [Google Scholar] [CrossRef]
- Irastorza, A.; Zarandona, I.; Andonegi, M.; Guerrero, P.; de la Caba, K. The versatility of collagen and chitosan: From food to biomedical applications. Food Hydrocoll. 2021, 116, 106633. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, M.; Jafarpour, A.; Undeland, I. Sequential extraction of gel-forming proteins, collagen and collagen hydrolysate from gutted silver carp (Hypophthalmichthys molitrix), a biorefinery approach. Food Chem. 2018, 242, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Balaure, P.C.; Holban, A.M.; Grumezescu, A.M.; Mogoşanu, G.D.; Bălşeanu, T.A.; Stan, M.S.; Dinischiotu, A.; Volceanov, A.; Mogoantă, L. In vitro and in vivo studies of novel fabricated bioactive dressings based on collagen and zinc oxide 3D scaffolds. Int. J. Pharm. 2019, 557, 199–207. [Google Scholar] [CrossRef]
- Liu, L.; Hu, D.; Han, X. A three-dimensional unit cell model with controllable crimped structure for investigating finite strain response of collagen fiber reinforced biological composites. Compos. Sci. Technol. 2018, 164, 178–186. [Google Scholar] [CrossRef]
- Lei, J.; Zou, B.; Zhang, R.; Zhang, K.; Xie, R.; Zhang, W.; Wu, J.; Li, S.; Zheng, B.; Huo, F. Regenerating leather waste for flexible pressure sensing applications. J. Leather Sci. Eng. 2019, 1, 7. [Google Scholar] [CrossRef]
- Peng, Z.; Zheng, S.; Zhang, X.; Yang, J.; Wu, S.; Ding, C.; Lei, L.; Chen, L.; Feng, G. Flexible Wearable Pressure Sensor Based on Collagen Fiber Material. Micromachines 2022, 13, 694. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Mun, S.; Ko, H.-U.; Zhai, L.; Kafy, A.; Kim, J. Renewable smart materials. Smart Mater. Struct. 2016, 25, 073001. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Asgharnasl, S.; Moghim Aliabadi, H.A.; Tahmasebi, B.; Radinekiyan, F.; Maleki, A.; Bahreinizad, H.; Mahdavi, M.; Alavijeh, M.S.; Saber, R.; et al. Magnetic graphene oxide–lignin nanobiocomposite: A novel, eco-friendly and stable nanostructure suitable for hyperthermia in cancer therapy. RSC Adv. 2022, 12, 3593–3601. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Grant, A.M.; Ma, R.; Zhang, S.; Tsukruk, V.V. R: Reports, Naturally-derived biopolymer nanocomposites: Interfacial design, properties and emerging applications. Mater. Sci. Eng. 2018, 125, 1–41. [Google Scholar] [CrossRef]
- Sharma, B.; Malik, P.; Jain, P. Biopolymer reinforced nanocomposites: A comprehensive review. Mater. Today Commun. 2018, 16, 353–363. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Ege, D.; Kamali, A.R.; Boccaccini, A.R. Graphene Oxide/Polymer-Based Biomaterials. Adv. Eng. Mater. 2017, 19, 1700627. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Biru, E.I.; Necolau, M.I.; Zainea, A.; Iovu, H. Graphene Oxide–Protein-Based Scaffolds for Tissue Engineering: Recent Advances and Applications. Polymers 2022, 14, 1032. [Google Scholar]
- Panzavolta, S.; Bracci, B.; Gualandi, C.; Focarete, M.L.; Treossi, E.; Kouroupis-Agalou, K.; Rubini, K.; Bosia, F.; Brely, L.; Pugno, N.M.; et al. Structural reinforcement and failure analysis in composite nanofibers of graphene oxide and gelatin. Carbon 2014, 78, 566–577. [Google Scholar] [CrossRef]
- Bazrafshan, Z.; Stylios, G.K. High Performance of Covalently Grafting onto Collagen in The Presence of Graphene Oxide. Nanomaterials 2018, 8, 703. [Google Scholar] [CrossRef] [PubMed]
- ASTM D 638-03; Standard Test Method for Tensile Properties of Plastics Annual Book of ASTM Standards. American Society of Testing and Materials: Philadelphia, PA, USA, 2003.
- Zhang, Y.; Chen, Z.; Liu, X.; Shi, J.; Chen, H.; Gong, Y. SEM, FTIR and DSC Investigation of Collagen Hydrolysate Treated Degraded Leather. J. Cult. Herit. 2021, 48, 205–210. [Google Scholar] [CrossRef]
- Badea, E.; Usacheva, T.; Della Gatta, G. The use of differential scanning calorimetry to characterise collagen deterioration in parchment. Ross. Khimicheskij Zhurnal 2015, 59, 28. [Google Scholar]
- Payne, K.J.; Veis, A. Fourier transform ir spectroscopy of collagen and gelatin solutions: Deconvolution of the amide I band for conformational studies. Biopolymers 1988, 27, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Belbachir, K.; Noreen, R.; Gouspillou, G.; Petibois, C. Collagen types analysis and differentiation by FTIR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 829–837. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J. Membr. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Surekha, G.; Krishnaiah, K.V.; Ravi, N.; Padma Suvarna, R. FTIR, Raman and XRD analysis of graphene oxide films prepared by modified Hummers method. J. Phys. Conf. Ser. 2020, 1495, 012012. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Guo, S.; Zhang, J. Interactions of graphene and graphene oxide with proteins and peptides. Nanotechnol. Rev. 2013, 2, 27–45. [Google Scholar] [CrossRef]
- Abhilash, V.; Rajender, N.; Suresh, K. Chapter 14—X-ray diffraction spectroscopy of polymer nanocomposites. In Spectroscopy of Polymer Nanocomposites; Thomas, S., Rouxel, D., Ponnamma, D., Eds.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 410–451. [Google Scholar]
- Meng, Z.; Zheng, X.; Tang, K.; Liu, J.; Ma, Z.; Zhao, Q. Dissolution and regeneration of collagen fibers using ionic liquid. Int. J. Biol. Macromol. 2012, 51, 440–448. [Google Scholar] [CrossRef]
- Ji, P.; Zhang, C.; Kong, Y.; Liu, H.; Guo, J.; Shi, L.; Yang, H.; Gu, Z.; Liu, Y. Collagen Film with Bionic Layered Structure and High Light Transmittance for Personalized Corneal Repair Fabricated by Controlled Solvent Evaporation Technique. J. Funct. Biomater. 2022, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; An, S.-J.; Lim, Y.-M.; Huh, J.-B. The Efficacy of Electron Beam Irradiated Bacterial Cellulose Membranes as Compared with Collagen Membranes on Guided Bone Regeneration in Peri-Implant Bone Defects. Materials 2017, 10, 1018. [Google Scholar] [CrossRef] [PubMed]
- Andonegi, M.; Irastorza, A.; Izeta, A.; Cabezudo, S.; de la Caba, K.; Guerrero, P. A Green Approach towards Native Collagen Scaffolds: Environmental and Physicochemical Assessment. Polymers 2020, 12, 1597. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Huang, X.; Gao, M.; Liao, X.; Shi, B. Polyphenol-grafted collagen fiber as reductant and stabilizer for one-step synthesis of size-controlled gold nanoparticles and their catalytic application to 4-nitrophenol reduction. Green Chem. 2011, 13, 651–658. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K.-H. Advances in Functional Biopolymer-Based Nanocomposites for Active Food Packaging Applications. Polymers 2021, 13, 4198. [Google Scholar] [CrossRef]
- Morimune, S.; Nishino, T.; Goto, T. Poly(vinyl alcohol)/graphene oxide nanocomposites prepared by a simple eco-process. Polym. J. 2012, 44, 1056–1063. [Google Scholar] [CrossRef]
- Poulin, P.; Jalili, R.; Neri, W.; Nallet, F.; Divoux, T.; Colin, A.; Aboutalebi, S.H.; Wallace, G.; Zakri, C. Superflexibility of graphene oxide. Proc. Natl. Acad. Sci. USA 2016, 113, 11088–11093. [Google Scholar] [CrossRef]
- Choi, S.-J.; Kim, S.-J.; Kim, I.-D. Ultrafast optical reduction of graphene oxide sheets on colorless polyimide film for wearable chemical sensors. NPG Asia Mater. 2016, 8, e315. [Google Scholar] [CrossRef]
- Mondal, R.K.; Dubey, K.A.; Bhardwaj, Y.K. Role of the interface on electron transport in electro-conductive polymer-matrix composite: A review. Polym. Compos. 2021, 42, 2614–2628. [Google Scholar] [CrossRef]
- Akamatsu, Y.; Tatsumisago, M.; Minami, T. Electrical Conductivity and Determination of Mobile Ion Species in the Glasses of the System ZrF4-BaF2-LiF. J. Ceram. Soc. Jpn. 1989, 97, 1109–1115. [Google Scholar] [CrossRef]
- Franco, M.; Alves, R.; Perinka, N.; Tubio, C.R.; Costa, P.; Lanceros-Mendéz, S. Water-Based Graphene Inks for All-Printed Temperature and Deformation Sensors. ACS Appl. Electron. Mater. 2020, 2, 2857–2867. [Google Scholar] [CrossRef]







| Films | Tg (°C) ± 1 °C | Td (°C) ± 1 °C | ΔH (mJ/g) ± 1% |
|---|---|---|---|
| Control | 46.9 | 94.2 | 275.7 |
| 0.25GO | 48.3 | 99.4 | 238.8 |
| 0.50GO | 48.6 | 99.1 | 227.5 |
| 0.75GO | 48.7 | 99.4 | 227.4 |
| 1GO | 48.9 | 100.5 | 219.9 |
| 3GO | 48.9 | 101.7 | 219.7 |
| Area (%) | C–C/C–H 284.6 (eV) ± 0.2 eV | C–O/C–N 286.1 (eV) ± 0.2 eV | C=O/N–C=O 287.5 (eV) ± 0.2 eV | C=N/C–N 399.5 (eV) ± 0.2 eV | O–C=O/O=C–N 531.1 (eV) ± 0.2 eV |
|---|---|---|---|---|---|
| Control | 69.5 | 3.1 | 9.3 | 5.9 | 12.2 |
| 1GO | 56.0 | 3.4 | 14.1 | 11.7 | 14.8 |
| Films | TS (MPa) | EAB (%) |
|---|---|---|
| Control | 33.2 ± 2.4 a | 6.0 ± 0.3 a |
| 0.25GO | 35.8 ± 1.3 a | 6.0 ± 0.3 a |
| 0.50GO | 38.9 ± 2.5 a | 6.0 ± 0.3 a |
| 0.75GO | 40.2 ± 2.2 b | 5.8 ± 0.3 a |
| 1GO | 42.0 ± 2.3 b,c | 5.5 ± 0.1 b |
| 3GO | 44.1 ± 1.0 c | 5.4 ± 0.1 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andonegi, M.; Correia, D.M.; Pereira, N.; Costa, C.M.; Lanceros-Mendez, S.; de la Caba, K.; Guerrero, P. Sustainable Collagen Composites with Graphene Oxide for Bending Resistive Sensing. Polymers 2023, 15, 3855. https://doi.org/10.3390/polym15193855
Andonegi M, Correia DM, Pereira N, Costa CM, Lanceros-Mendez S, de la Caba K, Guerrero P. Sustainable Collagen Composites with Graphene Oxide for Bending Resistive Sensing. Polymers. 2023; 15(19):3855. https://doi.org/10.3390/polym15193855
Chicago/Turabian StyleAndonegi, Mireia, Daniela M. Correia, Nelson Pereira, Carlos M. Costa, Senentxu Lanceros-Mendez, Koro de la Caba, and Pedro Guerrero. 2023. "Sustainable Collagen Composites with Graphene Oxide for Bending Resistive Sensing" Polymers 15, no. 19: 3855. https://doi.org/10.3390/polym15193855
APA StyleAndonegi, M., Correia, D. M., Pereira, N., Costa, C. M., Lanceros-Mendez, S., de la Caba, K., & Guerrero, P. (2023). Sustainable Collagen Composites with Graphene Oxide for Bending Resistive Sensing. Polymers, 15(19), 3855. https://doi.org/10.3390/polym15193855