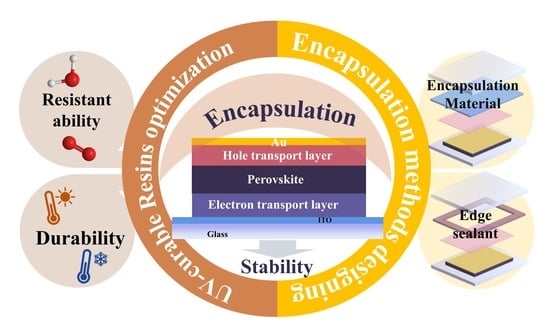
Recent Advances in UV-Cured Encapsulation for Stable and Durable Perovskite Solar Cell Devices
Abstract
:1. Introduction
2. Materials and Methods Used for PSC Encapsulation
2.1. The PSC Degradation Mechanism and the Requirements for PSC Encapsulation Materials
2.2. Comparison of Different Encapsulation Structures, Methods, and Materials
3. The Design of UV-Curable Resins for Highly Durable PSC Devices
3.1. The UV-Driven Curing Process
3.2. The Design of UV-Curable Resin for PSC Encapsulation
3.2.1. UV-Curable Resin Design to Reduce the Water Vapor Oxygen Transmission Rate
3.2.2. UV-Curable Resin Design to Retard Cracking and Aging
3.2.3. UV-Curable Resin Design to Reduce the Required UV Irradiation Dose
3.2.4. Commercially Available UV-Curable Resins as Candidates for PSC Encapsulation Materials
4. Recent Advances in Encapsulation Methods Using UV-Curable Resins as PSC Encapsulation
4.1. UV-Curable Resin Used as Encapsulation Materials

4.2. UV-Curable Resin Used as an Edge Sealant
4.3. UV-Curable Resin Used in Other Structures
4.4. Comparison between “Blanket-Cover” and “Edge-Sealant” Structures
5. Summary and Perspective
- To prevent the encapsulating adhesive from reacting with the core layers of the cells;
- To enhance the toughness of the UV-curable encapsulating adhesive to adapt to shrinkage deformation in the PSC encapsulation cover plate;
- To meet the application requirements in the encapsulation process of future flexible solar cells [157].
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Ma, F.; Qu, Z.; Yu, S.; Shen, T.; Deng, H.-X.; Chu, X.; Peng, X.; Yuan, Y.; Zhang, X.; et al. Inactive (Pb2)2RbCl stabilizes perovskite films for efficient solar cells. Science 2022, 377, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jeong, J.; Lu, H.; Lee, T.K.; Eickemeyer, F.T.; Liu, Y.; Choi, I.W.; Choi, S.J.; Jo, Y.; Kim, H.-B.; et al. Conformal quantum dot-SnO2 layers as electron transporters for efficient perovskite solar cells. Science 2022, 375, 302–306. [Google Scholar] [CrossRef]
- Laboratory, N.R.E. Best Research-Cell Efficiency. Available online: https://www.nrel.gov/pv/interactive-cell-efficiency.html# (accessed on 30 June 2023).
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.-K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.-P.; et al. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Mathews, I.; Sofia, S.; Ma, E.; Jean, J.; Laine, H.S.; Siah, S.C.; Buonassisi, T.; Peters, I.M. Economically Sustainable Growth of Perovskite Photovoltaics Manufacturing. Joule 2020, 4, 822–839. [Google Scholar] [CrossRef]
- Song, Z.; McElvany, C.L.; Phillips, A.B.; Celik, I.; Krantz, P.W.; Watthage, S.C.; Liyanage, G.K.; Apul, D.; Heben, M.J. A technoeconomic analysis of perovskite solar module manufacturing with low-cost materials and techniques. Energy Environ. Sci. 2017, 10, 1297–1305. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Wang, X.; Sun, Y.; Zhao, Z.; Li, Y.; Zhou, H.; Chen, Q. Cost Analysis of Perovskite Tandem Photovoltaics. Joule 2018, 2, 1559–1572. [Google Scholar] [CrossRef]
- Khatoon, S.; Kumar Yadav, S.; Chakravorty, V.; Singh, J.; Bahadur Singh, R.; Hasnain, M.S.; Hasnain, S.M.M. Perovskite solar cell’s efficiency, stability and scalability: A review. Mater. Sci. Energy Technol. 2023, 6, 437–459. [Google Scholar] [CrossRef]
- Kim, H.; Lim, J.; Park, S.; Song, S. A review on the engineering of hole-transporting materials for perovskite solar cells with high efficiency and high stability. Dye. Pigment. 2023, 15, 111449. [Google Scholar] [CrossRef]
- Cheacharoen, R.; Rolston, N.; Harwood, D.; Bush, K.A.; Dauskardt, R.H.; McGehee, M.D. Design and understanding of encapsulated perovskite solar cells to withstand temperature cycling. Energy Environ. Sci. 2018, 11, 144–150. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, Y.; Li, Z.; Zhang, L.; Ju, M.-G.; Luo, D.; Yang, Y.; Yang, M.; Kim, D.H.; Yang, W.; et al. Stable Formamidinium-Based Perovskite Solar Cells via In Situ Grain Encapsulation. Adv. Energy Mater. 2018, 8, 1800232. [Google Scholar] [CrossRef]
- Hwang, I.; Jeong, I.; Lee, J.; Ko, M.J.; Yong, K. Enhancing Stability of Perovskite Solar Cells to Moisture by the Facile Hydrophobic Passivation. ACS Appl. Mater. Interfaces 2015, 7, 17330–17336. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Ono, L.K.; Qi, Y. Advances and challenges to the commercialization of organic–inorganic halide perovskite solar cell technology. Mater. Today Energy 2018, 7, 169–189. [Google Scholar] [CrossRef]
- Dunfield, S.P.; Bliss, L.; Zhang, F.; Luther, J.M.; Zhu, K.; Hest, M.F.A.M.; Reese, M.O.; Berry, J.J. From Defects to Degradation: A Mechanistic Understanding of Degradation in Perovskite Solar Cell Devices and Modules. Adv. Energy Mater. 2020, 10, 1904054. [Google Scholar] [CrossRef]
- Zhang, F.; Bi, D.; Pellet, N.; Xiao, C.; Li, Z.; Berry, J.J.; Zakeeruddin, S.M.; Zhu, K.; Grätzel, M. Suppressing defects through the synergistic effect of a Lewis base and a Lewis acid for highly efficient and stable perovskite solar cells. Energy Environ. Sci. 2018, 11, 3480–3490. [Google Scholar] [CrossRef]
- Yang, J.; Siempelkamp, B.D.; Liu, D.; Kelly, T.L. Investigation of CH3NH3PbI3 Degradation Rates and Mechanisms in Controlled Humidity Environments Using in Situ Techniques. ACS Nano 2015, 9, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Christians, J.A.; Miranda Herrera, P.A.; Kamat, P.V. Transformation of the Excited State and Photovoltaic Efficiency of CH3NH3PbI3 Perovskite upon Controlled Exposure to Humidified Air. J. Am. Chem. Soc. 2015, 137, 1530–1538. [Google Scholar] [CrossRef]
- Boyd, C.C.; Cheacharoen, R.; Leijtens, T.; McGehee, M.D. Understanding Degradation Mechanisms and Improving Stability of Perovskite Photovoltaics. Chem. Rev. 2019, 119, 3418–3451. [Google Scholar] [CrossRef]
- Frost, J.M.; Butler, K.T.; Brivio, F.; Hendon, C.H.; van Schilfgaarde, M.; Walsh, A. Atomistic Origins of High-Performance in Hybrid Halide Perovskite Solar Cells. Nano Lett. 2014, 14, 2584–2590. [Google Scholar] [CrossRef]
- Mishra, A.K.; Shukla, R.K. Effect of humidity in the perovskite solar cell. Mater. Today Proc. 2020, 29, 836–838. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Z.; Huang, L.; Yue, G.; Liu, J.; Lu, X.; Hu, Z.; Shang, M.; Han, L.; Zhu, Y. Bifunctional alkyl chain barriers for efficient perovskite solar cells. Chem. Commun. 2015, 51, 7047–7050. [Google Scholar] [CrossRef]
- Tsai, H.; Asadpour, R.; Blancon, J.-C.; Stoumpos, C.C.; Durand, O.; Strzalka, J.W.; Chen, B.; Verduzco, R.; Ajayan, P.M.; Tretiak, S.; et al. Light-induced lattice expansion leads to high-efficiency perovskite solar cells. Science 2018, 360, 67–70. [Google Scholar] [CrossRef] [PubMed]
- deQuilettes, D.W.; Zhang, W.; Burlakov, V.M.; Graham, D.J.; Leijtens, T.; Osherov, A.; Bulović, V.; Snaith, H.J.; Ginger, D.S.; Stranks, S.D. Photo-induced halide redistribution in organic–inorganic perovskite films. Nat. Commun. 2016, 7, 11683. [Google Scholar] [CrossRef]
- Hoke, E.T.; Slotcavage, D.J.; Dohner, E.R.; Bowring, A.R.; Karunadasa, H.I.; McGehee, M.D. Reversible photo-induced trap formation in mixed-halide hybrid perovskites for photovoltaics. Chem. Sci. 2015, 6, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.-W.; Thilakan, A.P.; Li, J.-X.; Chen, T.-P.; Li, S.-S.; Chen, C.-W.; Yabushita, A.; Osada, M.; Tsukagoshi, K.; Sasaki, T. UV Degradation Mechanism of TiO2-Based Perovskite Solar Cells Studied by Pump-Probe Spectroscopy; SPIE: Berkeley, CA, USA, 2020; Volume 11366. [Google Scholar]
- Domanski, K.; Alharbi, E.A.; Hagfeldt, A.; Grätzel, M.; Tress, W. Systematic investigation of the impact of operation conditions on the degradation behaviour of perovskite solar cells. Nat. Energy 2018, 3, 61–67. [Google Scholar] [CrossRef]
- Kumar, A.; Bansode, U.; Ogale, S.; Rahman, A. Understanding the thermal degradation mechanism of perovskite solar cells via dielectric and noise measurements. Nanotechnology 2020, 31, 365403. [Google Scholar] [CrossRef]
- Zhang, D.; Li, D.; Hu, Y.; Mei, A.; Han, H. Degradation pathways in perovskite solar cells and how to meet international standards. Commun. Mater. 2022, 3, 58. [Google Scholar] [CrossRef]
- Kim, N.-K.; Min, Y.H.; Noh, S.; Cho, E.; Jeong, G.; Joo, M.; Ahn, S.-W.; Lee, J.S.; Kim, S.; Ihm, K.; et al. Investigation of Thermally Induced Degradation in CH3NH3PbI3 Perovskite Solar Cells using In-situ Synchrotron Radiation Analysis. Sci. Rep. 2017, 7, 4645. [Google Scholar] [CrossRef]
- Ma, S.; Yuan, G.; Zhang, Y.; Yang, N.; Li, Y.; Chen, Q. Development of encapsulation strategies towards the commercialization of perovskite solar cells. Energy Environ. Sci. 2022, 15, 13–55. [Google Scholar] [CrossRef]
- Schwenzer, J.A.; Rakocevic, L.; Gehlhaar, R.; Abzieher, T.; Gharibzadeh, S.; Moghadamzadeh, S.; Quintilla, A.; Richards, B.S.; Lemmer, U.; Paetzold, U.W. Temperature Variation-Induced Performance Decline of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 16390–16399. [Google Scholar] [CrossRef]
- Guo, R.; Khenkin, M.V.; Arnaoutakis, G.E.; Samoylova, N.A.; Barbé, J.; Lee, H.K.H.; Tsoi, W.C.; Katz, E.A. Initial Stages of Photodegradation of MAPbI3 Perovskite: Accelerated Aging with Concentrated Sunlight. Sol. RRL 2020, 4, 1900270. [Google Scholar] [CrossRef]
- Kempe, M.D. Ultraviolet light test and evaluation methods for encapsulants of photovoltaic modules. Sol. Energy Mater. Sol. Cells 2010, 94, 246–253. [Google Scholar] [CrossRef]
- Cros, S.; de Bettignies, R.; Berson, S.; Bailly, S.; Maisse, P.; Lemaitre, N.; Guillerez, S. Definition of encapsulation barrier requirements: A method applied to organic solar cells. Sol. Energy Mater. Sol. Cells 2011, 95, S65–S69. [Google Scholar] [CrossRef]
- Uddin, A.; Upama, M.B.; Yi, H.; Duan, L. Encapsulation of Organic and Perovskite Solar Cells: A Review. Coatings 2019, 9, 65. [Google Scholar] [CrossRef]
- Kim, J.-S.; Yang, S.; Bae, B.-S. Thermally Stable Transparent Sol−Gel Based Siloxane Hybrid Material with High Refractive Index for Light Emitting Diode (LED) Encapsulation. Chem. Mater. 2010, 22, 3549–3555. [Google Scholar] [CrossRef]
- Raman, R.K.; Gurusamy Thangavelu, S.A.; Venkataraj, S.; Krishnamoorthy, A. Materials, methods and strategies for encapsulation of perovskite solar cells: From past to present. Renew. Sustain. Energy Rev. 2021, 151, 111608. [Google Scholar] [CrossRef]
- Ligon-Auer, S.C.; Schwentenwein, M.; Gorsche, C.; Stampfl, J.; Liska, R. Toughening of photo-curable polymer networks: A review. Polym. Chem. 2016, 7, 257–286. [Google Scholar] [CrossRef]
- Ahmad, J.; Bazaka, K.; Anderson, L.J.; White, R.D.; Jacob, M.V. Materials and methods for encapsulation of OPV: A review. Renew. Sustain. Energy Rev. 2013, 27, 104–117. [Google Scholar] [CrossRef]
- Gao, H.; Han, C.; Wan, X.; Chen, Y. Recent progress in non-fused ring electron acceptors for high performance organic solar cells. Ind. Chem. Mater. 2023, 1, 60–78. [Google Scholar] [CrossRef]
- Khenkin, M.V.; Katz, E.A.; Abate, A.; Bardizza, G.; Berry, J.J.; Brabec, C.; Brunetti, F.; Bulović, V.; Burlingame, Q.; Di Carlo, A.; et al. Consensus statement for stability assessment and reporting for perovskite photovoltaics based on ISOS procedures. Nat. Energy 2020, 5, 35–49. [Google Scholar] [CrossRef]
- Velilla, E.; Jaramillo, F.; Mora-Seró, I. High-throughput analysis of the ideality factor to evaluate the outdoor performance of perovskite solar minimodules. Nat. Energy 2021, 6, 54–62. [Google Scholar] [CrossRef]
- Horváth, E.; Kollár, M.; Andričević, P.; Rossi, L.; Mettan, X.; Forró, L. Fighting Health Hazards in Lead Halide Perovskite Optoelectronic Devices with Transparent Phosphate Salts. ACS Appl. Mater. Interfaces 2021, 13, 33995–34002. [Google Scholar] [CrossRef] [PubMed]
- Runser, R.; Kodur, M.; Skaggs, J.H.; Cakan, D.N.; Foley, J.B.; Finn, M., III; Fenning, D.P.; Lipomi, D.J. Stability of Perovskite Films Encapsulated in Single- and Multi-Layer Graphene Barriers. ACS Appl. Energy Mater. 2021, 4, 10314–10322. [Google Scholar] [CrossRef]
- Ramasamy, E.; Karthikeyan, V.; Rameshkumar, K.; Veerappan, G. Glass-to-glass encapsulation with ultraviolet light curable epoxy edge sealing for stable perovskite solar cells. Mater. Lett. 2019, 250, 51–54. [Google Scholar] [CrossRef]
- Choi, E.Y.; Kim, J.; Lim, S.; Han, E.; Ho-Baillie, A.W.Y.; Park, N. Enhancing stability for organic-inorganic perovskite solar cells by atomic layer deposited Al2O3 encapsulation. Sol. Energy Mater. Sol. Cells 2018, 188, 37–45. [Google Scholar] [CrossRef]
- Zhao, J.; Brinkmann, K.O.; Hu, T.; Pourdavoud, N.; Becker, T.; Gahlmann, T.; Heiderhoff, R.; Polywka, A.; Görrn, P.; Chen, Y.; et al. Self-Encapsulating Thermostable and Air-Resilient Semitransparent Perovskite Solar Cells. Adv. Energy Mater. 2017, 7, 1602599. [Google Scholar] [CrossRef]
- Lv, Y.; Xu, P.; Ren, G.; Chen, F.; Nan, H.; Liu, R.; Wang, D.; Tan, X.; Liu, X.; Zhang, H.; et al. Low-Temperature Atomic Layer Deposition of Metal Oxide Layers for Perovskite Solar Cells with High Efficiency and Stability under Harsh Environmental Conditions. ACS Appl. Mater. Interfaces 2018, 10, 23928–23937. [Google Scholar] [CrossRef]
- Malekshahi Byranvand, M.; Behboodi-Sadabad, F.; Alrhman Eliwi, A.; Trouillet, V.; Welle, A.; Ternes, S.; Hossain, I.M.; Khan, M.R.; Schwenzer, J.A.; Farooq, A.; et al. Chemical vapor deposited polymer layer for efficient passivation of planar perovskite solar cells. J. Mater. Chem. A 2020, 8, 20122–20132. [Google Scholar] [CrossRef]
- Ocaña, L.; Montes, C.; González-Pérez, S.; González-Díaz, B.; Llarena, E. Characterization of a New Low Temperature Encapsulation Method with Ethylene-Vinyl Acetate under UV Irradiation for Perovskite Solar Cells. Appl. Sci. 2022, 12, 5228. [Google Scholar] [CrossRef]
- Jin, J.; Chen, S.; Zhang, J. Investigation of UV aging influences on the crystallization of ethylene-vinyl acetate copolymer via successive self-nucleation and annealing treatment. J. Polym. Res. 2010, 17, 827–836. [Google Scholar] [CrossRef]
- Sultan, B.-Å.; Sörvik, E. Thermal degradation of EVA and EBA—A comparison. II. Changes in unsaturation and side group structure. J. Appl. Polym. Sci. 1991, 43, 1747–1759. [Google Scholar] [CrossRef]
- Pern, F.J. Factors that affect the EVA encapsulant discoloration rate upon accelerated exposure. Sol. Energy Mater. Sol. Cells 1996, 41–42, 587–615. [Google Scholar] [CrossRef]
- Boyd, C.C.; Cheacharoen, R.; Bush, K.A.; Prasanna, R.; Leijtens, T.; McGehee, M.D. Barrier Design to Prevent Metal-Induced Degradation and Improve Thermal Stability in Perovskite Solar Cells. ACS Energy Lett. 2018, 3, 1772–1778. [Google Scholar] [CrossRef]
- Lin, B.; Zheng, C.; Zhu, Q.; Xie, F. A polyolefin encapsulant material designed for photovoltaic modules: From perspectives of peel strength and transmittance. J. Therm. Anal. Calorim. 2020, 140, 2259–2265. [Google Scholar] [CrossRef]
- Bush, K.A.; Palmstrom, A.F.; Yu, Z.J.; Boccard, M.; Cheacharoen, R.; Mailoa, J.P.; McMeekin, D.P.; Hoye, R.L.Z.; Bailie, C.D.; Leijtens, T.; et al. 23.6%-efficient monolithic perovskite/silicon tandem solar cells with improved stability. Nat. Energy 2017, 2, 17009. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Kim, B.; Byun, H.R.; Kim, S.H.; Oh, H.M.; Baik, S.; Jeong, M.S. Enhanced Stability of MAPbI3 Perovskite Solar Cells using Poly(p-chloro-xylylene) Encapsulation. Sci. Rep. 2019, 9, 15461. [Google Scholar] [CrossRef] [PubMed]
- Adothu, B.; Bhatt, P.D.; Kartikay, P.; Zele, S.; Costa, F.R.; Oderkerk, J.; Mallick, S. Determination of Crystallinity and Thermal Stability of Newly Developed Thermoplastic Polyolefin Encapsulant for the c-Si PV Module Application. In Proceedings of the 2019 IEEE 46th Photovoltaic Specialists Conference (PVSC), Chicago, IL, USA, 16–21 June 2019; pp. 0487–0490. [Google Scholar]
- Adothu, B.; Bhatt, P.; Chattopadhyay, S.; Zele, S.; Oderkerk, J.; Sagar, H.P.; Costa, F.R.; Mallick, S. Newly developed thermoplastic polyolefin encapsulant–A potential candidate for crystalline silicon photovoltaic modules encapsulation. Sol. Energy 2019, 194, 581–588. [Google Scholar] [CrossRef]
- Shi, L.; Bucknall, M.P.; Young, T.L.; Zhang, M.; Hu, L.; Bing, J.; Lee, D.S.; Kim, J.; Wu, T.; Takamure, N.; et al. Gas chromatography–mass spectrometry analyses of encapsulated stable perovskite solar cells. Science 2020, 368, eaba2412. [Google Scholar]
- Shi, L.; Young, T.L.; Kim, J.; Sheng, Y.; Wang, L.; Chen, Y.; Feng, Z.; Keevers, M.J.; Hao, X.; Verlinden, P.J.; et al. Accelerated Lifetime Testing of Organic–Inorganic Perovskite Solar Cells Encapsulated by Polyisobutylene. ACS Appl. Mater. Interfaces 2017, 9, 25073–25081. [Google Scholar] [CrossRef]
- Li, R.; Jing, Y.; Liu, X.; Xu, Y.; Wang, D.; Li, W.; Hou, W.; Wu, J.; Lan, Z. Stability enhancement of perovskite solar cells via multi-point ultraviolet-curing-based protection. J. Power Sources 2022, 520, 230906. [Google Scholar] [CrossRef]
- McKenna, B.; Troughton, J.R.; Watson, T.M.; Evans, R.C. Enhancing the stability of organolead halide perovskite films through polymer encapsulation. RSC Adv. 2017, 7, 32942–32951. [Google Scholar] [CrossRef]
- Jang, J.H.; Kim, B.-J.; Kim, J.-H.; Han, E.; Choi, E.-Y.; Ji, C.; Kim, K.T.; Kim, J.; Park, N. A Novel Approach for the Development of Moisture Encapsulation Poly(vinyl alcohol-co-ethylene) for Perovskite Solar Cells. ACS Omega 2019, 4, 9211–9218. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mukherjee, A.; Wu, H.; Yu, P.; Li, Y.; Hsieh, C. Accelerated Test for Light-Weight Photovoltaic Module Encapsulants. In Proceedings of the 2020 47th IEEE Photovoltaic Specialists Conference (PVSC), Calgary, AB, Canada, 15 June–21 August 2020; pp. 0707–0709. [Google Scholar]
- Fu, Z.; Xu, M.; Sheng, Y.; Yan, Z.; Meng, J.; Tong, C.; Li, D.; Wan, Z.; Ming, Y.; Mei, A.; et al. Encapsulation of Printable Mesoscopic Perovskite Solar Cells Enables High Temperature and Long-Term Outdoor Stability. Adv. Funct. Mater. 2019, 29, 1809129. [Google Scholar] [CrossRef]
- Søndergaard, R.R.; Makris, T.; Lianos, P.; Manor, A.; Katz, E.A.; Gong, W.; Tuladhar, S.M.; Nelson, J.; Tuomi, R.; Sommeling, P.; et al. The use of polyurethane as encapsulating method for polymer solar cells—An inter laboratory study on outdoor stability in 8 countries. Sol. Energy Mater. Sol. Cells 2012, 99, 292–300. [Google Scholar] [CrossRef]
- Ma, S.; Bai, Y.; Wang, H.; Zai, H.; Wu, J.; Li, L.; Xiang, S.; Liu, N.; Liu, L.; Zhu, C.; et al. 1000 h Operational Lifetime Perovskite Solar Cells by Ambient Melting Encapsulation. Adv. Energy Mater. 2020, 10, 1902472. [Google Scholar] [CrossRef]
- Khan, M.Z.H.; Al-Mamun, M.R.; Halder, P.K.; Aziz, M.A. Performance improvement of modified dye-sensitized solar cells. Renew. Sustain. Energy Rev. 2017, 71, 602–617. [Google Scholar] [CrossRef]
- Gong, J.W.; Sumathy, K.; Qiao, Q.Q.; Zhou, Z.P. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sustain. Energy Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Matteocci, F.; Cinà, L.; Lamanna, E.; Cacovich, S.; Divitini, G.; Midgley, P.A.; Ducati, C.; Di Carlo, A. Encapsulation for long-term stability enhancement of perovskite solar cells. Nano Energy 2016, 30, 162–172. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.; Baral, P.; Vijayaraghavan, S.N.; Yan, F.; Gong, X.; Wang, H. Blade-coated inverted perovskite solar cells in an ambient environment. Sol. Energy Mater. Sol. Cells 2022, 246, 111894. [Google Scholar] [CrossRef]
- Dong, Q.; Liu, F.; Wong, M.K.; Tam, H.W.; Djurišić, A.B.; Ng, A.; Surya, C.; Chan, W.K.; Ng, A.M.C. Encapsulation of Perovskite Solar Cells for High Humidity Conditions. ChemSusChem 2016, 9, 2597–2603. [Google Scholar] [CrossRef]
- Baranwal, A.K.; Kanda, H.; Shibayama, N.; Masutani, H.; Peiris, T.A.N.; Kanaya, S.; Segawa, H.; Miyasaka, T.; Ito, S. Thermal Degradation Analysis of Sealed Perovskite Solar Cell with Porous Carbon Electrode at 100 °C for 7000 h. Energy Technol. 2019, 7, 245–252. [Google Scholar] [CrossRef]
- Hoppe, C.E.; Williams, R.J.J. Self-Assembly of Epoxy-Based Polymers. In Epoxy Polymers; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 109–120. [Google Scholar]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Grant, L.; Yan, Z.; Nugen, T.; Weichun, C.; Yongzhi, H.; Shi, F.G. Materials Challenges and Solutions for the Packaging of High Power LEDs. In Proceedings of the 2006 11th International Symposium on Advanced Packaging Materials: Processes, Properties and Interface, Atlanta, GA, USA, 15–17 March 2006; p. 63. [Google Scholar]
- Kumar, R.N.; Keem, L.Y.; Mang, N.C.; Abubakar, A. Ultraviolet radiation curable epoxy resin encapsulant for light emitting diodes. J. Appl. Polym. Sci. 2006, 100, 1048–1056. [Google Scholar] [CrossRef]
- Yang, K.-Y.; Kim, J.; Cho, H.K.; Ha, T.-J.; Kim, Y.-H. Environment-stable solar window modules encapsulated with UV-curable transparent resin. Sol. Energy 2017, 158, 528–532. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Lee, K.-T.; Huang, W.-K.; Siao, H.-Y.; Chang, Y.-C. High-Performance, Air-Stable, Low-Temperature Processed Semitransparent Perovskite Solar Cells Enabled by Atomic Layer Deposition. Chem. Mater. 2015, 27, 5122–5130. [Google Scholar] [CrossRef]
- Kim, B.-J.; Jang, J.H.; Kim, J.; Oh, K.S.; Choi, E.Y.; Park, N. Efficiency and stability enhancement of organic–inorganic perovskite solar cells through micropatterned Norland Optical Adhesive and polyethylene terephthalate encapsulation. Mater. Today Commun. 2019, 20, 100537. [Google Scholar] [CrossRef]
- Kaltenbrunner, M.; Adam, G.; Głowacki, E.D.; Drack, M.; Schwödiauer, R.; Leonat, L.; Apaydin, D.H.; Groiss, H.; Scharber, M.C.; White, M.S.; et al. Flexible high power-per-weight perovskite solar cells with chromium oxide–metal contacts for improved stability in air. Nat. Mater. 2015, 14, 1032–1039. [Google Scholar] [CrossRef]
- Hashmi, S.G.; Martineau, D.; Li, X.; Ozkan, M.; Tiihonen, A.; Dar, M.I.; Sarikka, T.; Zakeeruddin, S.M.; Paltakari, J.; Lund, P.D.; et al. Air Processed Inkjet Infiltrated Carbon Based Printed Perovskite Solar Cells with High Stability and Reproducibility. Adv. Mater. Technol. 2017, 2, 1600183. [Google Scholar] [CrossRef]
- Gaddam, S.K.; Pothu, R.; Boddula, R. Advanced polymer encapsulates for photovoltaic devices—A review. J. Mater. 2021, 7, 920–928. [Google Scholar] [CrossRef]
- Chen, Z.; Chisholm, B.J.; Webster, D.C.; Zhang, Y.; Patel, S. Study of epoxidized-cardanol containing cationic UV curable materials. Prog. Org. Coat. 2009, 65, 246–250. [Google Scholar] [CrossRef]
- Moad, G.; Solomon, D.H. 2—Radical Reactions. In The Chemistry of Radical Polymerization, 2nd ed.; Moad, G., Solomon, D.H., Eds.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2005; pp. 11–48. [Google Scholar]
- Photopolymers: Photoresist Materials, Processes, and Applications Kenichiro Nakamura. MRS Bull. 2015, 40, 372. [CrossRef]
- Sangermano, M. UV-Cured Nanostructured Epoxy Coatings. In Epoxy Polymers; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 235–251. [Google Scholar]
- Chandra, R.; Soni, R.K. Recent developments in thermally curable and photocurable systems. Prog. Polym. Sci. 1994, 19, 137–169. [Google Scholar] [CrossRef]
- Podsiadły, R.; Podemska, K.; Szymczak, A.M. Novel visible photoinitiators systems for free-radical/cationic hybrid photopolymerization. Dye. Pigment. 2011, 91, 422–426. [Google Scholar] [CrossRef]
- Kim, H.; Ra, H.N.; Kim, M.; Kim, H.G.; Kim, S.S. Enhancement of barrier properties by wet coating of epoxy-ZrP nanocomposites on various inorganic layers. Prog. Org. Coat. 2017, 108, 25–29. [Google Scholar] [CrossRef]
- Islam, M.B.; Yanagida, M.; Shirai, Y.; Nabetani, Y.; Miyano, K. NiOx Hole Transport Layer for Perovskite Solar Cells with Improved Stability and Reproducibility. ACS Omega 2017, 2, 2291–2299. [Google Scholar] [CrossRef] [PubMed]
- Kovrov, A.; Helgesen, M.; Boeffel, C.; Kröpke, S.; Søndergaard, R.R. Novel acrylic monomers for organic photovoltaics encapsulation. Sol. Energy Mater. Sol. Cells 2020, 204, 110210. [Google Scholar] [CrossRef]
- Jung, K.; Bae, J.-Y.; Park, S.J.; Yoo, S.; Bae, B.-S. High performance organic-inorganic hybrid barrier coating for encapsulation of OLEDs. J. Mater. Chem. 2011, 21, 1977–1983. [Google Scholar] [CrossRef]
- Reyna, Y.; Salado, M.; Kazim, S.; Pérez-Tomas, A.; Ahmad, S.; Lira-Cantu, M. Performance and stability of mixed FAPbI3(0.85)MAPbBr3(0.15) halide perovskite solar cells under outdoor conditions and the effect of low light irradiation. Nano Energy 2016, 30, 570–579. [Google Scholar] [CrossRef]
- Castro-Hermosa, S.; Top, M.; Dagar, J.; Fahlteich, J.; Brown, T.M. Quantifying Performance of Permeation Barrier—Encapsulation Systems for Flexible and Glass-Based Electronics and Their Application to Perovskite Solar Cells. Adv. Electron. Mater. 2019, 5, 1800978. [Google Scholar] [CrossRef]
- Gao, N.; Liu, W.; Yan, Z.; Wang, Z. Synthesis and properties of transparent cycloaliphatic epoxy–silicone resins for opto-electronic devices packaging. Opt. Mater. 2013, 35, 567–575. [Google Scholar] [CrossRef]
- Park, J.H.; Baek, S.-D.; Cho, J.I.; Yoo, J.Y.; Yoon, S.-Y.; Kim, S.; Lee, S.; Kim, Y.S.; Myoung, J.-M. Characteristics of transparent encapsulation materials for OLEDs prepared from mesoporous silica nanoparticle-polyurethane acrylate resin composites. Compos. Part B Eng. 2019, 175, 107188. [Google Scholar] [CrossRef]
- Gonzalez, M.G.; Cabanelas, J.C.; Pozuelo, J.; Baselga, J. Preparation of cycloaliphatic epoxy hybrids with non-conventional amine-curing agents. J. Therm. Anal. Calorim. 2011, 103, 717–723. [Google Scholar] [CrossRef]
- Xing, Y.Q.; Chi, J.K.; Xiao, M. Reactive molecular dynamics simulation on the pyrolysis characteristics of epoxy resin under the effect of partial discharge active products. High Perform. Polym. 2021, 33, 635–645. [Google Scholar] [CrossRef]
- Wan, X.; Demir, B.; An, M.; Walsh, T.R.; Yang, N. Thermal conductivities and mechanical properties of epoxy resin as a function of the degree of cross-linking. Int. J. Heat Mass Transf. 2021, 180, 121821. [Google Scholar] [CrossRef]
- Valette, L.; Pascault, J.P.; Magny, B. Use of hydroxyl functionalized (meth)acrylic cross-linked polymer microparticles as chain transfer agent in cationic photopolymerization of cycloaliphatic epoxy monomer, 2. Macromol. Mater. Eng. 2003, 288, 762–770. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, C.; Yao, X.; Yang, X.; Zhang, W.; Zhang, G. Transparent thioether-ester-bridged polysilsesquioxane coating with enhanced water vapor barrier performance. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129739. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, C.; Cui, X.; Sun, J.; Ding, R.; Zhang, Q.; Xu, Y. Transparent and Dense Ladder-Like Alkylene-Bridged Polymethylsiloxane Coating with Enhanced Water Vapor Barrier Property. ACS Appl. Mater. Interfaces 2015, 7, 22157–22165. [Google Scholar] [CrossRef] [PubMed]
- Chiang, T.H.; Chen, C.H.; Liu, C.Y. Effect of sealing with ultraviolet-curable adhesives on the performance of dye-sensitized solar cells. J. Appl. Polym. Sci. 2015, 132, 42015. [Google Scholar] [CrossRef]
- Griffini, G.; Bella, F.; Nisic, F.; Dragonetti, C.; Roberto, D.; Levi, M.; Bongiovanni, R.; Turri, S. Multifunctional Luminescent Down-Shifting Fluoropolymer Coatings: A Straightforward Strategy to Improve the UV-Light Harvesting Ability and Long-Term Outdoor Stability of Organic Dye-Sensitized Solar Cells. Adv. Energy Mater. 2015, 5, 1401312. [Google Scholar] [CrossRef]
- Bella, F.; Griffini, G.; Correa-Baena, J.-P.; Saracco, G.; Gratzel, M.; Hagfeldt, A.; Turri, S.; Gerbaldi, C. Improving efficiency and stability of perovskite solar cells with photocurable fluoropolymers. Science 2016, 354, 203–206. [Google Scholar] [CrossRef]
- Yoon, B.; Park, C.-S.; Song, H.-J.; Kwak, J.; Lee, S.-S.; Lee, H. Perovskite solar cells integrated with blue cut-off filters for mitigating light-induced degradation. Opt. Express 2022, 30, 31367–31380. [Google Scholar] [CrossRef]
- Wang, Y.; Ahmad, I.; Leung, T.; Lin, J.; Chen, W.; Liu, F.; Ng, A.M.C.; Zhang, Y.; Djurišić, A.B. Encapsulation and Stability Testing of Perovskite Solar Cells for Real Life Applications. ACS Mater. Au 2022, 2, 215–236. [Google Scholar] [CrossRef] [PubMed]
- Weerasinghe, H.C.; Watkins, S.E.; Duffy, N.; Jones, D.J.; Scully, A.D. Influence of moisture out-gassing from encapsulant materials on the lifetime of organic solar cells. Sol. Energy Mater. Sol. Cells 2015, 132, 485–491. [Google Scholar] [CrossRef]
- Qiu, Y.; Qian, L.; Chen, Y.; Hao, J. Improving the fracture toughness and flame retardant properties of epoxy thermosets by phosphaphenanthrene/siloxane cluster-like molecules with multiple reactive groups. Compos. Part B: Eng. 2019, 178, 107481. [Google Scholar] [CrossRef]
- Mi, X.; Liang, N.; Xu, H.; Wu, J.; Jiang, Y.; Nie, B.; Zhang, D. Toughness and its mechanisms in epoxy resins. Prog. Mater. Sci. 2022, 130, 100977. [Google Scholar] [CrossRef]
- Morselli, D.; Bondioli, F.; Sangermano, M.; Messori, M. Photo-cured epoxy networks reinforced with TiO2 in-situ generated by means of non-hydrolytic sol–gel process. Polymer 2012, 53, 283–290. [Google Scholar] [CrossRef]
- Bao, Q.; Wang, B.; Liu, Y.; Wang, Q.; Yang, Z. Epoxy resin flame retarded and toughed via flexible siloxane chain containing phosphaphenanthrene. Polym. Degrad. Stab. 2020, 172, 109055. [Google Scholar] [CrossRef]
- Wang, L.; Liang, Y.; Yu, Q.; Chen, S.; Zhang, J.; Miao, M.; Zhang, D. Synthesis of epoxy-ended hyperbranched polyesters with reinforcing and toughening function for diglycidyl ether of bisphenol-A. Polym. Compos. 2018, 39, E2046–E2055. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lee, J.L. The synthesis, characterization, and photoinitiated cationic polymerization of silicon-containing epoxy resins. Polym. Mater Sci. Eng. Prepr. 1989, 60, 217–218. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, D.; Li, Z.; Lin, H.; Chen, X.; Oliver, S.; Shi, S.; Lei, L. Toughness modification of cationic UV-cured cycloaliphatic epoxy resin by hydroxyl polymers with different structures. Eur. Polym. J. 2020, 127, 109594. [Google Scholar] [CrossRef]
- Luo, L.; Meng, Y.; Qiu, T.; Li, X. An epoxy-ended hyperbranched polymer as a new modifier for toughening and reinforcing in epoxy resin. J. Appl. Polym. Sci. 2013, 130, 1064–1073. [Google Scholar] [CrossRef]
- Liu, W.; Tian, P.; Huang, Y.; Zhang, J. A novel UV-curable epoxy resin modified with cholic acid for high-frequency dielectric packaging. Prog. Org. Coat. 2022, 167, 106849. [Google Scholar] [CrossRef]
- Xiao, S.; Chen, Q.; Chen, M.; Hong, X. Research on the Epoxy-Acrylic Ester Hybrid UV-Curing System. J. High. Educ. Chem. 2002, 23, 1797–1800. [Google Scholar]
- Gao, N.; Liu, W.Q.; Ma, S.Q.; Yan, Z.L.; Zhao, Y. Modification of Epoxy Resin with Cycloaliphatic-Epoxy Oligosiloxane for Light-Emitting Diode (LED) Encapsulation Application. J. Macromol. Sci. Part B 2012, 51, 1509–1524. [Google Scholar] [CrossRef]
- Liu, R.; Xu, Y.; Jia, J.; Chen, P.; Zhang, F.; Zhang, L.; Chen, Y. Improvement on curing performance and morphology of E5I/TPGDA mixture in a free radical-cationic hybrid photopolymerization system. J. Polym. Res. 2020, 27, 166. [Google Scholar] [CrossRef]
- Scanone, A.C.; Casado, U.; Schroeder, W.F.; Hoppe, C.E. Visible-light photopolymerization of epoxy-terminated poly(dimethylsiloxane) blends: Influence of the cycloaliphatic monomer content on the curing behavior and network properties. Eur. Polym. J. 2020, 134, 109841. [Google Scholar] [CrossRef]
- Pupin, C.; Ross, A.; Dubois, C.; Rietsch, J.-C.; Ruiz, E. Predicting porosity formation in phenolic resins for RTM manufacturing: The porosity map. Compos. Part A Appl. Sci. Manuf. 2017, 100, 294–304. [Google Scholar] [CrossRef]
- Han, Y.; Meyer, S.; Dkhissi, Y.; Weber, K.; Pringle, J.M.; Bach, U.; Spiccia, L.; Cheng, Y.-B. Degradation observations of encapsulated planar CH3NH3PbI3 perovskite solar cells at high temperatures and humidity. J. Mater. Chem. A 2015, 3, 8139–8147. [Google Scholar] [CrossRef]
- Hossain, M.I.; Qarony, W.; Ma, S.; Zeng, L.; Knipp, D.; Tsang, Y.H. Perovskite/Silicon Tandem Solar Cells: From Detailed Balance Limit Calculations to Photon Management. Nano-Micro Lett. 2019, 11, 58. [Google Scholar] [CrossRef]
- Mansour Rezaei Fumani, N.; Arabpour Roghabadi, F.; Alidaei, M.; Sadrameli, S.M.; Ahmadi, V.; Najafi, F. Prolonged Lifetime of Perovskite Solar Cells Using a Moisture-Blocked and Temperature-Controlled Encapsulation System Comprising a Phase Change Material as a Cooling Agent. ACS Omega 2020, 5, 7106–7114. [Google Scholar] [CrossRef]
- Arabpour Roghabadi, F.; Mansour Rezaei Fumani, N.; Alidaei, M.; Ahmadi, V.; Sadrameli, S.M. High Power UV-Light Irradiation as a New Method for Defect Passivation in Degraded Perovskite Solar Cells to Recover and Enhance the Performance. Sci. Rep. 2019, 9, 9448. [Google Scholar] [CrossRef]
- Drobny, J.G. Radiation Technology for Polymers, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Zhou, R.; Pan, H.; Wan, D.; Malval, J.-P.; Jin, M. Bicarbazole-based oxime esters as novel efficient photoinitiators for photopolymerization under UV-Vis LEDs. Prog. Org. Coat. 2021, 157, 106306. [Google Scholar] [CrossRef]
- Chao, P.; Gu, R.; Ma, X.; Wang, T.; Zhao, Y. Thiophene-substituted phenothiazine-based photosensitisers for radical and cationic photopolymerization reactions under visible laser beams (405 and 455 nm) 11Electronic supplementary information (ESI) available. Polym. Chem. 2016, 7, 5147–5156. [Google Scholar] [CrossRef]
- Wong, C.P. Application of Polymer in Encapsulation of Electronic Parts; Electronic Applications: Berlin/Heidelberg, Germany, 1988; Springer: Berlin/Heidelberg, Germany, 1988; pp. 63–83. [Google Scholar]
- Rutsch, W.; Dietliker, K.; Leppard, D.; Köhler, M.; Misev, L.; Kolczak, U.; Rist, G. Recent developments in photoinitiators. Prog. Org. Coat. 1996, 27, 227–239. [Google Scholar] [CrossRef]
- Shao, J.; Huang, Y.; Fan, Q. Visible light initiating systems for photopolymerization: Status, development and challenges. Polym. Chem. 2014, 5, 4195–4210. [Google Scholar] [CrossRef]
- Chiang, T.H.; Hsieh, T.E. A study of monomer’s effect on adhesion strength of UV-curable resins. Int. J. Adhes. Adhes. 2006, 26, 520–531. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, S.; Zhou, X.; Yang, Z.; Yuan, T. A novel multi-functional bio-based reactive diluent derived from cardanol for high bio-content UV-curable coatings application. Prog. Org. Coat. 2020, 148, 105880. [Google Scholar] [CrossRef]
- Rudawska, A.; Worzakowska, M.; Bociąga, E.; Olewnik-Kruszkowska, E. Investigation of selected properties of adhesive compositions based on epoxy resins. Int. J. Adhes. Adhes. 2019, 92, 23–36. [Google Scholar] [CrossRef]
- Cazin, I.; Gleirscher, M.O.; Fleisch, M.; Berer, M.; Sangermano, M.; Schlögl, S. Spatially controlling the mechanical properties of 3D printed objects by dual-wavelength vat photopolymerization. Addit. Manuf. 2022, 57, 102977. [Google Scholar] [CrossRef]
- Wu, S.-C.; Strover, L.T.; Yao, X.; Chen, X.-Q.; Xiao, W.-J.; Liu, L.-N.; Wang, J.; Visoly-Fisher, I.; Katz, E.A.; Li, W.-S. UV-Cross-linkable Donor–Acceptor Polymers Bearing a Photostable Conjugated Backbone for Efficient and Stable Organic Photovoltaics. ACS Appl. Mater. Interfaces 2018, 10, 35430–35440. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Y.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. Direct Contact of Selective Charge Extraction Layers Enables High-Efficiency Molecular Photovoltaics. Joule 2018, 2, 1108–1117. [Google Scholar] [CrossRef]
- Khadka, D.B.; Shirai, Y.; Yanagida, M.; Miyano, K. Degradation of encapsulated perovskite solar cells driven by deep trap states and interfacial deterioration. J. Mater. Chem. C 2018, 6, 162–170. [Google Scholar] [CrossRef]
- Jiang, Y.; Qiu, L.; Juarez-Perez, E.J.; Ono, L.K.; Hu, Z.; Liu, Z.; Wu, Z.; Meng, L.; Wang, Q.; Qi, Y. Reduction of lead leakage from damaged lead halide perovskite solar modules using self-healing polymer-based encapsulation. Nat. Energy 2019, 4, 585–593. [Google Scholar] [CrossRef]
- Kaufman, H.S. Handbook of epoxy resins. Henry Lee and Kris Neville. J. Appl. Polym. Sci. 1970, 14, 253. [Google Scholar] [CrossRef]
- Cho, M.; Jeon, G.G.; Sang, M.; Kim, T.S.; Suh, J.; Shin, S.J.; Choi, M.J.; Kim, H.W.; Kim, K.; Lee, J.Y.; et al. Ultra-thin thermally grown silicon dioxide nanomembrane for waterproof perovskite solar cells. J. Power Sources 2023, 563, 232810. [Google Scholar] [CrossRef]
- Wong-Stringer, M.; Game, O.S.; Smith, J.A.; Routledge, T.J.; Alqurashy, B.A.; Freestone, B.G.; Parnell, A.J.; Vaenas, N.; Kumar, V.; Alawad, M.O.A.; et al. High-Performance Multilayer Encapsulation for Perovskite Photovoltaics. Adv. Energy Mater. 2018, 8, 1801234. [Google Scholar] [CrossRef]
- Belich, N.A.; Petrov, A.A.; Ivlev, P.A.; Udalova, N.N.; Pustovalova, A.A.; Goodilin, E.A.; Tarasov, A.B. How to stabilize standard perovskite solar cells to withstand operating conditions under an ambient environment for more than 1000 hours using simple and universal encapsulation. J. Energy Chem. 2023, 78, 246–252. [Google Scholar] [CrossRef]
- Guarnera, S.; Abate, A.; Zhang, W.; Foster, J.M.; Richardson, G.; Petrozza, A.; Snaith, H.J. Improving the Long-Term Stability of Perovskite Solar Cells with a Porous Al2O3 Buffer Layer. J. Phys. Chem. Lett. 2015, 6, 432–437. [Google Scholar] [CrossRef]
- Tripathi, N.; Yanagida, M.; Shirai, Y.; Masuda, T.; Han, L.; Miyano, K. Hysteresis-free and highly stable perovskite solar cells produced via a chlorine-mediated interdiffusion method. J. Mater. Chem. A 2015, 3, 12081–12088. [Google Scholar] [CrossRef]
- Wu, Y.; Xie, F.; Chen, H.; Yang, X.; Su, H.; Cai, M.; Zhou, Z.; Noda, T.; Han, L. Thermally Stable MAPbI3 Perovskite Solar Cells with Efficiency of 19.19% and Area over 1 cm2 achieved by Additive Engineering. Adv. Mater. 2017, 29, 1701073. [Google Scholar] [CrossRef]
- Dong, F.; Xiong, T.; Yan, S.; Wang, H.; Sun, Y.; Zhang, Y.; Huang, H.; Wu, Z. Facets and Defects Cooperatively Promote Visible Light Plasmonic Photocatalysis With Bi Nanowires@BiOCl Nanosheets. J. Catal. 2016, 344, 401–410. [Google Scholar] [CrossRef]
- Liu, F.; Dong, Q.; Wong, M.K.; Djurišić, A.B.; Ng, A.; Ren, Z.; Shen, Q.; Surya, C.; Chan, W.K.; Wang, J.; et al. Is Excess PbI2 Beneficial for Perovskite Solar Cell Performance? Adv. Energy Mater. 2016, 6, 1502206. [Google Scholar] [CrossRef]
- Lertngim, A.; Phiriyawirut, M.; Wootthikanokkhan, J.; Yuwawech, K.; Sangkhun, W.; Kumnorkaew, P.; Muangnapoh, T. Preparation of Surlyn films reinforced with cellulose nanofibres and feasibility of applying the transparent composite films for organic photovoltaic encapsulation. R. Soc. Open Sci. 2017, 4, 170792. [Google Scholar] [CrossRef] [PubMed]
- Seethamraju, S.; Ramamurthy, P.C.; Madras, G. Organic passivation layer on flexible Surlyn substrate for encapsulating organic photovoltaics. Appl. Phys. Lett. 2014, 105, 104102. [Google Scholar] [CrossRef]
- Grancini, G.; Roldán-Carmona, C.; Zimmermann, I.; Mosconi, E.; Lee, X.; Martineau, D.; Narbey, S.; Oswald, F.; De Angelis, F.; Graetzel, M.; et al. One-Year stable perovskite solar cells by 2D/3D interface engineering. Nat. Commun. 2017, 8, 15684. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, A.K.; Kanaya, S.; Peiris, T.A.N.; Mizuta, G.; Nishina, T.; Kanda, H.; Miyasaka, T.; Segawa, H.; Ito, S. 100 °C Thermal Stability of Printable Perovskite Solar Cells Using Porous Carbon Counter Electrodes. ChemSusChem 2016, 9, 2604–2608. [Google Scholar] [CrossRef] [PubMed]
- Krauklis, A.E.; Echtermeyer, A.T. Mechanism of Yellowing: Carbonyl Formation during Hygrothermal Aging in a Common Amine Epoxy. Polymers 2018, 10, 1017. [Google Scholar] [CrossRef]
- Zhou, Z.; Yuan, Z.; Yin, Z.; Xue, Q.; Li, N.; Huang, F. Progress of semitransparent emerging photovoltaics for building integrated applications. Green Energy Environ. 2023. [Google Scholar] [CrossRef]













| Characteristics | Specifications |
|---|---|
| WVTR (water vapor transmission rate) | 10−4–10−6 g m−2 day−1 |
| OTR (oxygen transmission rate) | 10−3–10−5 cm3 m−2 day−1 atm−1 |
| Glass transition temperature (Tg) | <−40 °C (during winter in deserts) |
| Total light transmission | >90% of incident light |
| Water absorption | <0.5 wt% (20 °C/100% RH) |
| Tensile modulus | <20.7 MPa (<3000 psi) at 25 °C |
| UV absorption degradation | None (>350 nm) |
| Total hemispherical light transmission over the wavelength range from 400 nm to 1100 nm | >90% of incident light |
| Chemical inertness | No reaction (with embedded Cu coupons at 90 °C) |
| Resistance to thermal oxidation | Stable (up to 85 °C) |
| Hydrolysis | None (80 °C, 100% RH) |
| Mechanical creep | None (90 °C) |
| Hazing or clouding | None (80 °C, 100% RH) |
| Encapsulation Materials | WVTR (g/m2/Day) | Processing Time and Pressure | Processing Temp. | Refs. |
|---|---|---|---|---|
| EVA | ~28.0 | Lamination 15–30 min at vacuum 100 kPa | ~140–~150 °C | [66,67] |
| POE | ~3.8 | Lamination 15–30 min at vacuum 100 kPa | ~90–~130 °C | [66] |
| PET | 1 × 10−2 to 1 × 10−3 | Used as substrate and applied to the PSC with UV-curable epoxy | RT | [80,81] |
| PIB | 1 × 10−2 to 1 × 10−3 | Hot-pressed with a background pressure of 300–400 mTorr for ~10 min | RT-~160 °C | [61,66] |
| PMMA | ~55.2 | Solution spin-coating and 5 min on hot plate | ~80 °C | [63] |
| EVOH | ~4.72 × 10−2 | Precursor solution heated on a hot plate to form EVOH film and adhesive with UV-curable epoxy | ~70 °C | [64] |
| TPU | N/A | Spray-painted PU resin or vacuum 100 kPa crosslink at RT for ~24 h | RT-~110 °C | [66,82] |
| UV-cured resin | 102 to 10−1 | UV light 30–45 mW cm−2 illuminate 10 s–10 min | RT | [45,61,71] |
| Resin Composition | Structure | Pros | Ref |
|---|---|---|---|
| Transparent cycloaliphatic epoxy–silicone |  | Higher transmittances, lower water absorptions, better UV/thermal resistance and thermal stabilities | [97] |
| Transparent polyurethane acrylate–mesoporous silica nanoparticles composites |  | High transparency and low haze, low moisture permeability and high adhesive force | [98] |
| Ethyleneglycoldimethacrylate-bridged polysilsesquioxane |  | Better water vapor barrier property and adhesion property | [103] |
| Cycloaliphatic epoxy-oligosiloxane | See Figure 4b | High optical transparency, low permeability | [94] |
| Aliphatic hydrophobic backbone mixed 2-(perfluorohexyl) ethyl methacrylate | Mixture of CN991 with  | Lower surface energy and a better resistance to corrosion. | [105] |
| 2-isocyanatoethyl methacrylate and 2-isocyanatoethyl methacrylate |  with EuD4TEA with EuD4TEA | Down-shifting material to convert UV photons into valuable visible light and high hydrophobic | [106] |
| Epoxy resins–cycloaliphatic moieties bearing epoxy rings |  | Improved toughness | [116] |
| Cycloaliphatic epoxy-oligosiloxane-hyperbranched polyester terminated—six hydroxy-branched groups and poly(tetramethylene ether glycol) | See Figure 6b | Enhance the toughness and impact strength | [117] |
| Epoxy cresol novolac modified with cholic acid and glycidyl methacrylate |  | High glass transition temperature and low dielectric constant | [119] |
| Manufacturer | Model | Product Type | Tg | Ingredients | WVTR (g/m2/Day) | Refs. of Implementation |
|---|---|---|---|---|---|---|
| Three Bond Holdings Co., Ltd. (Tokyo, Japan) | Threebond 3035B | Acrylic resin | N/A | Silica 45–55 wt%; acrylate monomers, acrylate oligomers, photoinitiators, additives 50–60 wt% | 97 (@85 °C, 85%RH) | [68,71,95,140] |
| Ossila (Sheffield, UK) | Ossila E131 | Carboxylic acids epoxy resin | 138 °C | Epoxy resin 96 wt%, photoinitiators > 3 wt%, photostabilizers > 3 wt% | N/A | [95,96] |
| Nagase ChemeteX (Osaka, Japan) | XNR5516ZD | Epoxy | 102 °C | Epoxy resin 60–70 wt%; inorganic filler 30–40 wt% | 20 (@60 °C, 90%RH) | [61,92,141] |
| Electron-lite Corp. (Bethel, MN, USA) | ELC-2500 | Epoxy | N/A | N/A | N/A | [80] |
| Asahi Glass Corporation (Tokyo, Japan) | Lumiflon LF-910LM | Fluoropolymers | 37 °C | Fluoroethylene-alkylvinyl ether alternative copolymer | N/A | [107] |
| Panacol (Frankfurt, Germany) | Vitralit | Epoxy | >150 °C | Epoxy 80 wt%; SiO2 20 wt% | N/A | [45] |
| Norland Products Inc. (Jamesburg, NJ, USA) | NOA 63 | Urethane related resin | N/A | Mercapto esters 50–75 wt%; Triallyl isocyanuarte 15–40 wt% | N/A | [64,81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, M.; Ji, W.; Chao, C.; Li, J.; Dai, F.; Fan, X. Recent Advances in UV-Cured Encapsulation for Stable and Durable Perovskite Solar Cell Devices. Polymers 2023, 15, 3911. https://doi.org/10.3390/polym15193911
Cao M, Ji W, Chao C, Li J, Dai F, Fan X. Recent Advances in UV-Cured Encapsulation for Stable and Durable Perovskite Solar Cell Devices. Polymers. 2023; 15(19):3911. https://doi.org/10.3390/polym15193911
Chicago/Turabian StyleCao, Mengyu, Wenxi Ji, Cong Chao, Ji Li, Fei Dai, and Xianfeng Fan. 2023. "Recent Advances in UV-Cured Encapsulation for Stable and Durable Perovskite Solar Cell Devices" Polymers 15, no. 19: 3911. https://doi.org/10.3390/polym15193911
APA StyleCao, M., Ji, W., Chao, C., Li, J., Dai, F., & Fan, X. (2023). Recent Advances in UV-Cured Encapsulation for Stable and Durable Perovskite Solar Cell Devices. Polymers, 15(19), 3911. https://doi.org/10.3390/polym15193911