Electrochemical Comparison of 2D-Flexible Solid-State Supercapacitors Based on a Matrix of PVA/H3PO4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Electrodes
2.2.1. Dip-Coating (DC) of Lignin
2.2.2. Hydrothermal Growth of CuO Nanoparticles
2.2.3. Surface Morphology and Area Characterization
2.3. Electrochemical Characterization of Electrodes
2.4. FSC Fabrication
2.5. Electrochemical Characterization of FSCs
3. Results and Discussion
3.1. Electrodes Modification and Characterization
3.2. Flexible Supercapacitors’ Electrochemical Characterization
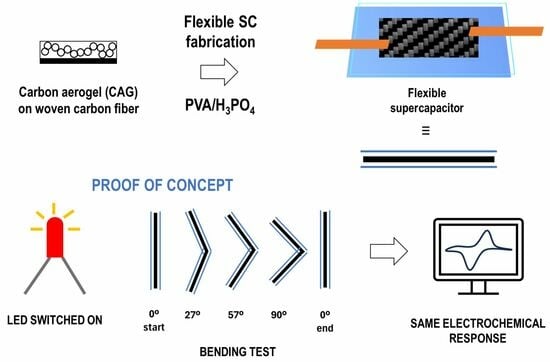
3.3. Bending Tests
3.4. Proof of Concept
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mao, M.; Hu, J.; Liu, H. Graphene-based materials for flexible electrochemical energy storage. Int. J. Energy Res. 2015, 39, 727–740. [Google Scholar] [CrossRef]
- Keum, K.; Kim, J.W.; Hong, S.Y.; Son, J.G.; Lee, S.-S.; Ha, J.S. Flexible/Stretchable Supercapacitors with Novel Functionality for Wearable Electronics. Adv. Mater. 2020, 32, 2002180. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Shi, T.; Zhan, X.; Long, H.; Xi, S.; Hu, H.; Tang, Z. High-performance all-solid-state flexible supercapacitors based on two-step activated carbon cloth. J. Power Sources 2014, 272, 16–23. [Google Scholar] [CrossRef]
- Xue, Q.; Sun, J.; Huang, Y.; Zhu, M.; Pei, Z.; Li, H.; Wang, Y.; Li, N.; Zhang, H.; Zhi, C. Recent progress on flexible and wearable supercapacitors. Small 2017, 13, 1701827. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Qian, Q.; Wei, L.; Jiang, W.; Goh, K.; Wei, J.; Chen, Y. Emergence of fiber supercapacitors. Chem. Soc. Rev. 2015, 44, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Karahan, H.E.; Wei, L.; Qian, Q.; Harris, A.T.; Minett, A.I.; Chen, Y. Textile energy storage: Structural design concepts, material selection and future perspectives. Energy Storage Mater. 2016, 3, 123–139. [Google Scholar] [CrossRef]
- Lv, Z.; Tang, Y.; Zhu, Z.; Wei, J.; Li, W.; Xia, H.; Jiang, Y.; Liu, Z.; Luo, Y.; Ge, X.; et al. Honeycomb-lantern-inspired 3D stretchable supercapacitors with enhanced specific areal capacitance. Adv. Mater. 2018, 30, 1805468. [Google Scholar] [CrossRef]
- Xie, P.; Yuan, W.; Liu, X.; Peng, Y.; Yin, Y.; Li, Y.; Wu, Z. Advanced carbon nanomaterials for state-of-the-art flexible supercapacitors. Energy Storage Mater. 2021, 36, 56–76. [Google Scholar] [CrossRef]
- Yu, G.; Hu, L.; Vosgueritchian, M.; Wang, H.; Xie, X.; McDonough, J.R.; Bao, Z. Solution-Processed Graphene/MnO2 Nanostructured Textiles for High-Performance Electrochemical Capacitors. Nano Lett. 2011, 11, 2905–2911. [Google Scholar] [CrossRef]
- Liu, W.; Lu, C.; Li, H.; Tay, R.Y.; Sun, L.; Wang, X.; Yu, A. Based all-solid-state flexible micro-supercapacitors with ultra-high rate and rapid frequency response capabilities. J. Mater. Chem. A 2016, 4, 3754–3764. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, Z.S.; Yang, S.; Dong, R.; Feng, X.; Müllen, K. Ultraflexible in-plane micro-supercapacitors by direct printing of solution-processable electrochemically exfoliated graphene. Adv. Mater. 2016, 28, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, S.; Vongehr, S.; Ali Syed, J.; Wang, X.; Meng, X. High-performance flexible solid-state carbon cloth supercapacitors based on highly processible N-graphene doped polyacrylic acid/polyaniline composites. Sci. Rep. 2016, 6, 12883. [Google Scholar] [CrossRef] [PubMed]
- Dubal, D.P.; Chodankar, N.R.; Kim, D.H.; Gomez-Romero, P. Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem. Soc. Rev. 2018, 47, 2065–2129. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Li, N.; Jin, S.; Yang, G.; Wang, C. All-Solid-State Symmetric Supercapacitor Based on Co3O4 Nanoparticles on Vertically Aligned Graphene. ACS Nano 2015, 9, 5310–5317. [Google Scholar] [CrossRef]
- Javaid, A.; Zafrullah, M.B.; Khan, F.; Bhatti, G.M. Improving the multifunctionality of structural supercapacitors by interleaving graphene nanoplatelets between carbon fibers and solid polymer electrolyte. J. Compos. Mater. 2018, 53, 1401–1409. [Google Scholar] [CrossRef]
- Hu, W.; Xiang, R.; Lin, J.; Cheng, Y.; Lu, C. Lignocellulosic Biomass Derived-Carbon Electrodes fro Flexible Supercapacitor: An Overview. Materials 2021, 14, 4571. [Google Scholar] [CrossRef]
- Szabó, L.; Imanishi, S.; Tetsuo, F.; Hirose, D.; Ueda, H.; Tsukegi, T.; Ninomiya, K.; Takahashi, K. Lignin as a functional Green Coating on Carbon Fiber Surface to Improve Interfacial Adhesion in Carbon Fiber Reinforced Polymers. Materials 2019, 12, 159. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Lu, X.; Ling, Y.; Yu, M.; Zhai, T.; Li, Y. Solid-state supercapacitor based on activated carbon cloths exhibits excellent rate capability. Adv. Mater. 2014, 26, 2676–2682. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, J. Carbon nanotube network film directly grown on carbon cloth for high-performance solid-state flexible supercapacitors. Nanotechnology 2014, 25, 035402. [Google Scholar] [CrossRef]
- Yu, M.; Zhai, T.; Lu, X.; Chen, X.; Xie, S.; Li, W.; Tong, Y. Manganese dioxide nanorod arrays on carbon fabric for flexible solid-state supercapacitors. J. Power Sources 2013, 239, 64–71. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, J.; Wang, X.; Liu, B.; Hou, X.; Yu, G.; Wang, P.; Chen, D.; Shen, G. Core–Shell CuCo2O4@MnO2 Nanowires on Carbon Fabrics as High-Performance Materials for Flexible, All-Solid-State, Electrochemical Capacitors. ChemElectroChem 2014, 1, 559–564. [Google Scholar] [CrossRef]
- Liao, Q.; Li, N.; Jin, S.; Yang, G.; Wang, C. A flexible all-solid-state asymmetric supercapacitors based on hierarchical carbon cloth@ CoMoO4@ NiCo layered double hydroxide core-shell heterostructures. Chem. Eng. J. 2018, 352, 29–38. [Google Scholar]
- Majumdar, D.; Ghosh, S. Recent advancements of copper oxide-based nanomaterials for supercapacitor applications. J. Energy Storage 2021, 34, 101995. [Google Scholar] [CrossRef]
- Gao, Y.; Zheng, S.; Fu, H.; Ma, J.; Xu, X.; Guan, L.; Wu, H.; Wu, Z.-S. Three-dimensional nitrogen doped hierarchically porous carbon aerogels with ultrahigh specific surface area for high-performance supercapacitors and flexible micro-supercapacitors. Carbon 2020, 168, 701–709. [Google Scholar] [CrossRef]
- Qian, H.; Kucernak, A.R.; Greenhalgh, E.S.; Bismarck, A.; Shaffer, M.S.P. Multifunctional structural supercapacitor composites based on carbon aerogel modified high performance carbon fiber fabric. ACS Appl. Mater. Interfaces 2013, 5, 6113–6122. [Google Scholar] [CrossRef]
- Deka, B.K.; Hazarika, A.; Kim, J.; Kim, N.; Jeong, H.E.; Park, Y.B.; Park, H.W. Bimetallic copper cobalt selenide nanowire-anchored woven carbon fiber-based structural supercapacitors. Chem. Eng. J. 2019, 355, 551–559. [Google Scholar] [CrossRef]
- Kong, K.; Deka, B.K.; Seo, J.W.; Park, Y.-B.; Park, H.W. Effect of CuO nanostructure morphology on the mechanical properties of CuO/woven carbon fiber/vinyl ester composites. Compos. Part A 2015, 78, 48–59. [Google Scholar] [CrossRef]
- Artigas-Arnaudas, J.; Muñoz, B.K.; Sánchez, M.; de Prado, J.; Utrilla, M.V.; Ureña, A. Surface modifications of carbon fiber electrodes for structural supercapacitors. Appl. Compos. Mater. 2022, 29, 889–900. [Google Scholar] [CrossRef]
- Yokoyama, S.; Motomiya, K.; Jeyadevan, B.; Tohji, K. Environmentally friendly synthesis and formation mechanism of copper nanowires with controlled aspect ratios from aqueous solution with ascorbic acid. J. Colloid Interface Sci. 2018, 531, 109–118. [Google Scholar] [CrossRef]
- Yuan, L.; Lu, X.-H.; Xiao, X.; Zhai, T.; Dai, J.; Zhang, F.; Hu, B.; Wang, X.; Gong, L.; Chen, J.; et al. Flexible Solid-State Supercapacitors Based on Carbon Nanoparticles/MnO2 Nanorods Hybrid Structure. ACS Nano 2012, 6, 656–661. [Google Scholar] [CrossRef]
- Qi, G.; Nguyen, S.; Anthony, D.B.; Kucernak, A.R.J.; Shaffer, M.S.P.; Greenhalgh, E.S. The influence of fabrication parameters on the electrochemical performance of multifunctional structural supercapacitors. Multifunct. Mater. 2021, 4, 034001. [Google Scholar] [CrossRef]
- Deka, B.K.; Hazarika, A.; Kim, J.; Park, Y.-B.; Park, H.W. Multifunctional CuO nanowire embodied structural supercapacitor based on woven carbon fiber/ionic liquid–polyester resin. Compos. Part A Appl. Sci. Manuf. 2016, 87, 256–262. [Google Scholar] [CrossRef]
- Zuo, W.; Li, R.; Zhou, C.; Li, Y.; Xia, J.; Liu, J. Battery-supercapacitor hybrid devices: Recent progress and future prospects. Adv. Sci. 2017, 4, 1600539. [Google Scholar] [CrossRef] [PubMed]
- Shirshova, N.; Qian, H.; Houllé, M.; Steinke, J.H.; Kucernak, A.R.; Fontana, Q.P.; Greenhalgh, E.S.; Bismarck, A.; Shaffer, M.S.P. Multifunctional structural energy storage composite supercapacitors. Faraday Discuss. 2014, 172, 81–103. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Yoon, C.-M.; Kim, Y.K.; Jang, J. High performance asymmetric supercapacitor twisted from carbon fiber/MnO2 and carbon fiber/MoO3. Carbon 2017, 116, 470–478. [Google Scholar] [CrossRef]
- Shen, C.; Xie, Y.; Zhu, B.; Sanghadasa, M.; Tang, Y.; Lin, L. Wearable woven supercapacitor fabrics with high energy density and load-bearing capability. Sci. Rep. 2017, 7, 14324. [Google Scholar] [CrossRef]
- Zhang, T.; Kim, C.H.; Cheng, Y.; Ma, Y.; Zhang, H.; Liu, J. Making a commercial carbon fiber cloth having comparable capacitances to carbon nanotubes and graphene in supercapacitors through a “top-down” approach. Nanoscale 2015, 7, 3285–3291. [Google Scholar] [CrossRef]
- Senokos, E.; Anthony, D.B.; Rubio Carrero, N.; Crespo Ribadeneyra, M.; Greenhalgh, E.S.; Shaffer, M.S.P. Robust Single-Walled Carbon Nanotube-Infiltrated Carbon Fiber Electrodes for Structural Supercapacitors: From Reductive Dissolution to High Performance Devices. Adv. Funct. Mater. 2023, 33, 2212697. [Google Scholar] [CrossRef]
- Greenhalgh, E.S.; Ankersen, J.; Asp, L.E.; Bismarck, A.; Fontana, Q.P.V.; Houlle, M.; Kalinka, G.; Kucernak, A.; Mistry, M.; Nguyen, S.; et al. Mechanical, electrical and microstructural characterisation of multifunctional structural power composites. J. Compos. Mater. 2015, 49, 1823. [Google Scholar] [CrossRef]
- Pernice, M.F.; Qi, G.; Senokos, E.; Anthony, D.B.; Nguyen, S.; Valkova, M.; Greenhalgh, E.S.; Shaffer, M.S.P.; Kucernak, A.R.J. Mechanical, electrochemical and multifunctional performance of a CFRP/carbon aerogel structural supercapacitor and its corresponding monofunctional equivalents. Multifunct. Mater. 2022, 5, 025002. [Google Scholar] [CrossRef]
- Yue, C.; Bismarck, A.; Qian, H. Carbon Aerogel Coated Carbon Fibre Fabrics as Electrode Materials for Multifunctional Supercapacitors. Master’s Thesis, Imperial College London, London, UK, 2011. [Google Scholar]






| Entry | Sample | SBET (m2/gC+cover) | Csp (F/gC+cover) |
|---|---|---|---|
| 1 | WCF | 0.244 | 0.19 |
| 2 | CuO | 0.853 | 5.42 |
| 3 | CAG | 63.47 | 1.7 |
| 4 | LIG | 0.278 | 0.18 |
| SCF | Csp (mF/cm2) | Csp (F/g) | σ (mS cm−1) | Rs (Ω) | Rs + Rct (Ω) |
|---|---|---|---|---|---|
| FSC-WCF | - | 0.023 | - | - | - |
| FSC-CuO | 12.1 | 0.328 | 2.08 | 2.95 | 10.61 |
| FSC-CAG | 9.6 | 0.259 | 1.44 | 8.26 | 69.10 |
| FSC-LIG | 0.14 | 0.004 | 0.034 | 164.9 | 414.68 |
| Separator | Rs/Rs + Rct (Ω) | Csp (F/g) | P (W/kg) | E (Wh/kg) |
|---|---|---|---|---|
| FSC-FP | 8.26/69.1 | 0.563 | 0.815 | 0.048 |
| FSC-WGF | 5.48/8.48 | 1.369 | 0.962 | 0.161 |
| Device | Capacitance (F) | Areal C (F/cm2) | Rs/Rct (W) |
|---|---|---|---|
| FSC1 | 2.14 | 0.143 | 1.02/1.14 |
| FSC2 | 2.00 | 0.134 | 1.13/1.36 |
| Double FSC | 4.66 | 0.311 | 0.6/1.13 |
| Composites | Electrolyte | Electrodes (F/g) | Capacitor | Reference |
|---|---|---|---|---|
| WCF | 3 M KCl | 0.02 | - | This work |
| WCF | 3 M KCl | 0.06 | - | [25] |
| a-WCF | 3 M | 2.63 | - | [25] |
| a-CFC | 6 M KCl | 197 (0.1 A/g) | 0.5 Fcm−2 | [37] |
| ACF | PVA-H3PO4 | 18.6 | 300 mFcm−2 | [36] |
| WCF-CNT | EMIMTFSI | 13.26 | 0.010 | [38,39] |
| WCF-CNT | 3 M KCl | 0.015 | [28] | |
| WCF-GNP | 3 M KCl | 0.405 | [28] | |
| WCF/CAG | EMIMTFSI | 2.4 | - | [31] |
| WCF/CAG | EMIMTFSI | 11.4 | 1.74 Fg−1; 135 mFcm−2 | [40] |
| WCF/CAG | Epoxy resin/EMIMTFSI | - | 0.212 Fg−1 | [40] |
| WCF/CAG | 3 M KCl | 5.9 | - | [25] |
| CF/CAG | EMIMTFSI | - | 1.49 Fg−1 | [25] |
| CF/CAG | EMIMTFSI | - | 0.75 Fg−1 | [41] |
| WCF/CAG | 3 M KCl | 4.35 | 1.4 Fg−1 | This work |
| WCF/CuO | 3 M KCl | 5.42 | 0.33 Fg−1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, B.K.; González-Banciella, A.; Ureña, D.; Sánchez, M.; Ureña, A. Electrochemical Comparison of 2D-Flexible Solid-State Supercapacitors Based on a Matrix of PVA/H3PO4. Polymers 2023, 15, 4036. https://doi.org/10.3390/polym15204036
Muñoz BK, González-Banciella A, Ureña D, Sánchez M, Ureña A. Electrochemical Comparison of 2D-Flexible Solid-State Supercapacitors Based on a Matrix of PVA/H3PO4. Polymers. 2023; 15(20):4036. https://doi.org/10.3390/polym15204036
Chicago/Turabian StyleMuñoz, Bianca K., Andrés González-Banciella, Daniel Ureña, María Sánchez, and Alejandro Ureña. 2023. "Electrochemical Comparison of 2D-Flexible Solid-State Supercapacitors Based on a Matrix of PVA/H3PO4" Polymers 15, no. 20: 4036. https://doi.org/10.3390/polym15204036
APA StyleMuñoz, B. K., González-Banciella, A., Ureña, D., Sánchez, M., & Ureña, A. (2023). Electrochemical Comparison of 2D-Flexible Solid-State Supercapacitors Based on a Matrix of PVA/H3PO4. Polymers, 15(20), 4036. https://doi.org/10.3390/polym15204036