Effect of Pulsed Electric Field Treatment on the Protein, Digestibility, and Physicochemical Properties of Starch Granules in Wheat Flour
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PEF Treatment of Wheat Flour
2.3. Scanning Electron Microscopy (SEM)
2.4. Confocal Laser Scanning Microscopy (CLSM)
2.5. Pasting Properties
2.6. Differential Scanning Calorimetry (DSC)
2.7. X-ray Diffraction (XRD)
2.8. Fourier-Transform Infrared Spectrometer (FTIR)
2.9. Particle Size Distribution
2.10. In Vitro Starch Digestibility of Flour
2.11. Statistical Analysis
3. Results
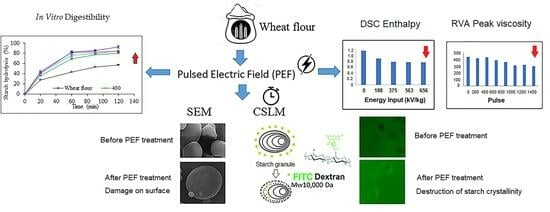
3.1. Scanning Electron Microscopy (SEM)
3.2. Confocal Laser Scanning Microscopy (CLSM)
3.3. Pasting Properties
3.4. Thermal Properties
3.5. X-ray Diffraction (XRD)
3.6. Fourier-Transform Infrared Spectrometer (FTIR)
3.7. Particle Size Distribution
3.8. In Vitro Starch Digestibility
4. Conclusions
Author Contributions
Funding
International Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merte, B.; Knorr, D. Development of non-thermal process for food preservation. Food Technol. 1992, 46, 124–133. [Google Scholar]
- Gilliland, S.E.; Speck, M.L. Inactivation of microorganisms by electrohydraulic shock. Appl. Microbiol. 1967, 15, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Sale, A.J.H.; Hamilton, W.A. Effects of high electric fields on microorganisms I. Killing of bacteria and yeast. Biochim. Biophys. Acta Gen. Subj. 1967, 148, 781–788. [Google Scholar] [CrossRef]
- Sale, A.J.H.; Hamilton, W.A. Effects of high electric fields on microorganisms III. Lysis of erythrocytes and protoplast. Biochim. Biophys. Acta Biomembr. 1968, 163, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Mahnič-Kalamiza, S.; Miklavčič, D. The phenomenon of electroporation. In Pulsed Electric Fields Technology for the Food Industry; Springer: Berlin/Heidelberg, Germany, 2022; pp. 107–141. [Google Scholar] [CrossRef]
- Han, Z.; Zeng, X.A.; Fu, N.; Yu, S.J.; Chen, X.D.; Kennedy, J.F. Effects of pulsed electric field treatments on some properties of tapioca starch. Carbohydr. Polym. 2012, 89, 1012–1017. [Google Scholar] [CrossRef]
- Li, Q.; Wu, Q.Y.; Jiang, W.; Qian, J.Y.; Zhang, L.; Wu, M.; Rao, S.Q.; Wu, C.S. Effect of pulsed electric field on structural properties and digestibility of starches with different crystalline types in solid state. Carbohydr. Polym. 2019, 207, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Duque, S.M.M.; Leong, S.Y.; Agyei, D.; Singh, J.; Larsen, N.; Oey, I. Understanding the impact of pulsed electric fields treatment on the thermal and pasting properties of raw and thermally processed oat flours. Food Res. Int. 2020, 129, 108839. [Google Scholar] [CrossRef]
- Nowosad, K.; Sujka, M.; Pankiewicz, U.; Kowalski, R. The application of PEF technology in food processing and human nutrition. J. Food Sci. Technol. 2021, 58, 397–411. [Google Scholar] [CrossRef]
- Singh, H.; Blennow, A.; Gupta, A.D.; Kaur, P.; Dhillon, B.; Sodhi, N.S.; Dubey, P.K. Pulsed light, pulsed electric field and cold plasma modification of starches: Technological advancements & effects on functional properties. J. Food Meas. Charact. 2022, 16, 4092–4109. [Google Scholar] [CrossRef]
- Zhu, F. Modifications of starch by electric field based techniques. Trends Food Sci. Technol. 2018, 75, 158–169. [Google Scholar] [CrossRef]
- Bluhm, H.; Schultheiss, C.; Frey, W.; Gusbeth, C.; Sack, M.; Strassner, R. Industrial scale treatment of biological cells with pulsed electric fields. In Proceedings of the Conference Record of the International Power Modulator Symposium and High Voltage Workshop, San Francisco, CA, USA, 23–26 May 2004; pp. 8–14. [Google Scholar]
- Grimi, N.; Mamouni, F.; Lebovka, N.; Vorobiev, E.; Vaxelaire, J. Acoustic impulse response in apple tissues treated by pulsed electric field. Biosyst. Eng. 2010, 105, 266–272. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Castanha, N.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Starch modification through environmentally friendly alternatives: A review. Crit. Rev. Food Sci. Nut. 2021, 61, 2482–2505. [Google Scholar] [CrossRef] [PubMed]
- Jeyamkondan, S.; Jayas, D.S.; Holley, R.A. Pulsed electric field processing of foods: A review. J. Food Prot. 1999, 62, 1088–1096. [Google Scholar] [CrossRef]
- Han, Z.; Zeng, X.A.; Yu, S.J.; Zhang, B.S.; Chen, X.D. Effects of pulsed electric fields (PEF) treatment on physicochemical properties of potato starch. Innov. Food Sci. Emerg. Technol. 2009, 10, 481–485. [Google Scholar] [CrossRef]
- Han, Z.; Yu, Q.; Zeng, X.A.; Luo, D.H.; Yu, S.J.; Zhang, B.S.; Chen, X.D. Studies on the microstructure and thermal properties of pulsed electric fields (PEF)-treated maize starch. Int. J. Food Eng. 2012, 8, 1168–1174. [Google Scholar] [CrossRef]
- Han, Z.; Zeng, X.A.; Zhang, B.S.; Yu, S.J. Effects of pulsed electric fields (PEF) treatment on the properties of corn starch. J. Food Eng. 2009, 93, 318–323. [Google Scholar] [CrossRef]
- Zeng, F.; Gao, Q.-Y.; Han, Z.; Zeng, X.-A.; Yu, S.-J. Structural properties and digestibility of pulsed electric field treated waxy rice starch. Food Chem. 2016, 194, 1313–1319. [Google Scholar] [CrossRef]
- Qiu, S.; Abbaspourrad, A.; Padilla-Zakour, O.I. Changes in the glutinous rice grain and physicochemical properties of its starch upon moderate treatment with pulsed electric field. Foods 2021, 10, 395. [Google Scholar] [CrossRef]
- Wu, C.; Wu, Q.-Y.; Wu, M.; Jiang, W.; Qian, J.-Y.; Rao, S.-Q.; Zhang, L.; Li, Q.; Zhang, C. Effect of pulsed electric field on properties and multi-scale structure of japonica rice starch. LWT Food Sci. Technol. 2019, 116, 108515. [Google Scholar] [CrossRef]
- Duque, S.M.M. Impact of Pulsed Electric Fields Treatment on Oat Flour Properties. Ph.D. Thesis, University of Otago, Dunedin, New Zealand, 2020. Available online: http://hdl.handle.net/10523/10461 (accessed on 1 June 2023).
- Abduh, S.B.M.; Leong, S.Y.; Agyei, D.; Oey, I. Understanding the properties of starch in potatoes (Solanum tuberosum var. Agria) after being treated with pulsed electric field processing. Foods 2019, 8, 159. [Google Scholar] [CrossRef]
- Yu, Y.-Q.; Li, S.-N.; Sun, C.-R.; Wei, S.L.; Jia, X.-Z.; Zeng, X.-A.; Zhang, B.; Haung, Q. Pulsed electric field combined with enzymatic hydrolysis improves the preparation efficiency and oil absorption of porous starch. Mod. Food Sci. Technol. 2021, 37, 157–163. [Google Scholar] [CrossRef]
- Hong, J.; An, D.; Zeng, X.-A.; Han, Z.; Zheng, X.; Cai, M.; Bian, K.; Aadil, R.M. Behaviors of large A-type and small B-type wheat starch granules esterified by conventional and pulsed electric fields assisted methods. Int. J. Biol. Macromol. 2020, 155, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.M.; Rosell, C.M.; Gómez, M. Modification of wheat flour functionality and digestibility through different extrusion conditions. J. Food Eng. 2014, 143, 74–79. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Y.-H.; Zhao, X.-D.; Zhang, L.; Li, Q.; Wu, C.; Ding, X.; Qian, J.-Y. Assessment of impact of pulsed electric field on functional, rheological and structural properties of vital wheat gluten. LWT Food Sci. Technol. 2021, 147, 111536. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Z.; Wang, X.; Sun, B.; Ma, S.; Wang, F. A promising strategy for mechanically modified wheat flour by milling of wheat endosperm. J. Cereal Sci. 2022, 104, 103440. [Google Scholar] [CrossRef]
- Achayuthakan, P.; Suphantharika, M.; BeMiller, J.N. Confocal laser scanning microscopy of dextran-rice starch mixtures. Carbohydr. Polym. 2012, 87, 557–563. [Google Scholar] [CrossRef]
- Eliasson, A.-C.; Gudmundsson, M.; Svensson, G. Thermal behavior of wheat starch in flour relation to flour quality. LWT Food Sci. Technol. 1995, 28, 227–235. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr. 1992, 46, S33–S50. [Google Scholar]
- Zhang, C.; Lyu, X.; Arshad, R.N.; Aadil, R.M.; Tong, Y.; Zhao, W.; Yang, R. Pulsed electric field as a promising technology for solid foods processing: A review. Food Chem. 2023, 403, 134367. [Google Scholar] [CrossRef]
- Castro, L.M.G.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M. Starch extraction and modification by pulsed electric fields. Food Rev. Int. 2021, 39, 2161–2182. [Google Scholar] [CrossRef]
- Prabhu, M.S.; Levkov, K.; Livney, Y.D.; Israel, A.; Golberg, A. High-voltage pulsed electric field preprocessing enhances extraction of starch, proteins, and ash from marine macroalgae Ulva ohnoi. ACS Sustain. Chem. Eng. 2019, 7, 17453–17463. [Google Scholar] [CrossRef]
- Xu, D.; Guan, W.; Wu, F.; Jin, Y.; Yang, N.; Jin, Z.; Xu, X. Improvement of baked wheat chips quality by protease-mediated enzymatic hydrolysis of wheat flour. LWT Food Sci. Technol. 2022, 157, 113043. [Google Scholar] [CrossRef]
- Kim, H.-S.; Huber, K.C. Channels with soft wheat starch A- and B-type granules. J. Cereal Sci. 2008, 48, 159–172. [Google Scholar] [CrossRef]
- Ma, M.; Chen, X.; Zhou, R.; Li, H.; Sui, Z.; Corke, H. The surface microstructure of rice starch is altered by the removal of granule-associated proteins. Food Hydrocoll. 2021, 121, 107038. [Google Scholar] [CrossRef]
- Zaidul, I.S.M.; Yamauchi, H.; Matsuura-Endo, C.; Takigawa, S.; Noda, T. Thermal analysis of mixtures of wheat flour and potato starches. Food Hydrocoll. 2008, 22, 499–504. [Google Scholar] [CrossRef]
- Eliasson, A.-C. Differential scanning calorimetry studies on wheat starch-gluten mixtures: I. Effect of gluten on the gelatinization of wheat starch. J. Cereal Sci. 1983, 1, 199–205. [Google Scholar] [CrossRef]
- Tiga, B.H.; Kumcuoglu, S.; Vatansever, M.; Tavman, S. Thermal and pasting properties of Quinoa-wheat flour blends and their effects on production of extruded instant noodles. J. Cereal Sci. 2021, 97, 103120. [Google Scholar] [CrossRef]
- Chen, D.J.; McClements, Z.P.; Zhang, R.J.; Zhang, X.L.; Bian, Z.Y.; Jin, Y.Q.; Tian, Y. Effect of pullulan on oil absorption and structural organization of native maize starch during frying. Food Chem. 2020, 309, 125681. [Google Scholar] [CrossRef]
- Zoau, X.; Wang, X.; Peng, P.; Ma, Q.; Zhang, X.; Zou, L.; Zhou, J.; Hu, X. Glutenin-gliadin ratio changes combining heat-moisture treatment significantly influences the in vitro digestibility of starch in recombinant wheat flours. Int. J. Biol. Macromol. 2023, 248, 125920. [Google Scholar] [CrossRef]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrared spectroscopy as a tool to characterise starch ordered structure—A joint FTIR–ATR, NMR, XRD and DSC study. Carbohydr. Polym. 2016, 139, 35–42. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Pataro, G.; Ferrari, G.; Augusto, P.E.D.; Le-Bali, P.; Le-Bali, A. Pulsed electric fields (PEF) treatment to enhance starch 3D printing application: Effect on structure, properties, and functionality of wheat and cassava starches. Innov. Food Sci. Emerg. Tecnol. 2021, 68, 102602. [Google Scholar] [CrossRef]
- Shen, H.; Yan, M.; Liu, X.; Ge, X.; Zeng, J.; Gao, H.; Zhang, G.; Li, W. Wheat starch particle size distribution regulates the dynamic transition behavior of gluten at different stages of dough mixing. Int. J. Biol. Macromol. 2023, 244, 125371. [Google Scholar] [CrossRef] [PubMed]
- Corgneau, M.; Gaiani, C.; Petit, J.; Nikolova, Y.; Banon, S.; Ritié-Pertusa, L.; Thanh Lam Le, D.; Scher, J. Digestibility of common native starches with reference to starch granule size, shape and surface features towards guidelines for starch-containing food products. Int. J. Food Sci. Technol. 2019, 54, 2132–2140. [Google Scholar] [CrossRef]








| Pulse Number | Specific Energy Input (kJ/kg) | Peak Viscosity | Trough | Breakdown | Final Viscosity | Setback | Pasting Temperature |
|---|---|---|---|---|---|---|---|
| (mPa·s) | (mPa·s) | (mPa·s) | (mPa·s) | (mPa·s) | (°C) | ||
| 0 | 0 | 440 ± 2 a | 280 ± 2 a | 160 ± 0 a | 730 ± 4 a | 450 ± 2 a | 81.01 ± 0.15 b |
| 200 | 94 | 431 ± 2 b | 275 ± 3 b | 156 ± 1 b | 690 ± 3 b | 429 ± 0 b | 81.20 ± 0.12 b |
| 400 | 188 | 415 ± 1 c | 268 ± 2 c | 147 ± 1 c | 675 ± 3 c | 421 ± 1 c | 81.12 ± 0.20 b |
| 600 | 282 | 390 ± 2 d | 249 ± 2 d | 141 ± 4 c | 647 ± 3 d | 398 ± 4 c | 81.20 ± 0.13 b |
| 800 | 375 | 364 ± 2 e | 241 ± 2 e | 123 ± 1 d | 631 ± 3 e | 390 ± 1 d | 82.10 ± 0.20 b |
| 1000 | 469 | 311 ± 2 g | 208 ± 3 f | 102 ± 2 e | 545 ± 3 g | 337 ± 1 f | 82.95 ± 0.10 a |
| 1200 | 563 | 320 ± 2 f | 215 ± 3 g | 105 ± 1 e | 568 ± 3 f | 353 ± 1 e | 82.85 ± 0.17 a |
| 1400 | 656 | 298 ± 1 h | 200 ± 3 h | 97 ± 2 f | 521 ± 2 g | 320 ± 5 g | 82.90 ± 0.05 a |
| Specific Energy Input (kJ/kg) | To (°C) | Tp (°C) | Tc (°C) | Tc − To (°C) | Enthalpy (kJ/kg) |
|---|---|---|---|---|---|
| 0 | 59.19 ± 0.19 a | 64.67 ± 0.44 a | 70.50 ± 0.54 a | 11.31 ± 0.35 a | 4.24 ± 0.15 a |
| 188 | 58.74 ± 0.30 a | 63.84 ± 0.47 a | 70.01 ± 0.04 a | 11.27 ± 0.14 a | 3.26 ± 0.60 b |
| 375 | 57.95 ± 0.65 a | 62.05 ± 1.20 ab | 67.23 ± 0.04 b | 9.28 ± 0.61 b | 2.84 ± 0.03 c |
| 563 | 58.54 ± 0.45 a | 62.12 ± 0.26 b | 68.04 ± 0.46 b | 9.50 ± 0.01 b | 2.81 ± 0.00 c |
| 656 | 58.25 ± 0.08 a | 62.00 ± 0.10 b | 66.80 ± 0.40 c | 8.55 ± 0.32 c | 2.73 ± 0.00 d |
| Electric Field Strength (kJ/kg) | 1047/1022 | 995/1022 |
|---|---|---|
| 0 | 0.744 ± 0.012 a | 1.017 ± 0.004 a |
| 188 | 0.729 ± 0.002 b | 1.015 ± 0.008 ab |
| 281 | 0.719 ± 0.008 b | 1.013 ± 0.012 b |
| 375 | 0.715 ± 0.004 b | 1.012 ± 0.003 b |
| 469 | 0.710 ± 0.010 c | 1.010 ± 0.002 c |
| 656 | 0.708 ± 0.004 c | 1.009 ± 0.005 c |
| Specific Energy Input (kJ/kg) | RDS (%) | SDS (%) | RS (%) |
|---|---|---|---|
| 0 | 27.59 ± 0.12 e | 29.68 ± 0.48 d | 42.73 ± 0.42 a |
| 188 | 36.51 ± 0.48 d | 43.74 ± 0.45 b | 9.75 ± 0.36 b |
| 375 | 40.50 ± 0.52 c | 44.13 ± 0.42 b | 15.37 ± 0.27 c |
| 563 | 44.56 ± 0.25 a | 39.08 ± 1.03 c | 16.36 ± 0.86 c |
| 656 | 41.72 ± 1.12 b | 51.00 ± 2.50 a | 7.28 ± 2.43 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achayuthakan, P.; Wongsagonsup, R.; Sriprablom, J.; Suphantharika, M.; Intra, P. Effect of Pulsed Electric Field Treatment on the Protein, Digestibility, and Physicochemical Properties of Starch Granules in Wheat Flour. Polymers 2023, 15, 4087. https://doi.org/10.3390/polym15204087
Achayuthakan P, Wongsagonsup R, Sriprablom J, Suphantharika M, Intra P. Effect of Pulsed Electric Field Treatment on the Protein, Digestibility, and Physicochemical Properties of Starch Granules in Wheat Flour. Polymers. 2023; 15(20):4087. https://doi.org/10.3390/polym15204087
Chicago/Turabian StyleAchayuthakan, Piyada, Rungtiwa Wongsagonsup, Jiratthitikan Sriprablom, Manop Suphantharika, and Panich Intra. 2023. "Effect of Pulsed Electric Field Treatment on the Protein, Digestibility, and Physicochemical Properties of Starch Granules in Wheat Flour" Polymers 15, no. 20: 4087. https://doi.org/10.3390/polym15204087
APA StyleAchayuthakan, P., Wongsagonsup, R., Sriprablom, J., Suphantharika, M., & Intra, P. (2023). Effect of Pulsed Electric Field Treatment on the Protein, Digestibility, and Physicochemical Properties of Starch Granules in Wheat Flour. Polymers, 15(20), 4087. https://doi.org/10.3390/polym15204087