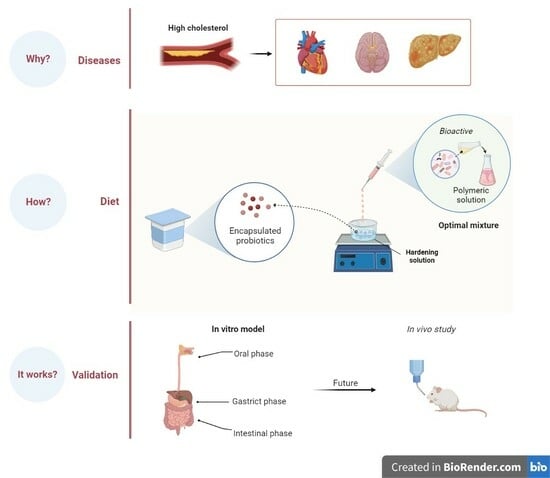
Probiotic Encapsulation: Bead Design Improves Bacterial Performance during In Vitro Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Strain and Bacterial Conservation
2.2.2. Biomass Production
2.3. Formulation of Wall Materials
2.3.1. Preparing Solutions
2.3.2. Mixture Design
2.4. Probiotic Encapsulation
2.4.1. Cell Release
2.4.2. Cell Viability
2.4.3. Encapsulation Efficiency (EE)
2.5. Characterization of Beads
2.5.1. Attenuated Total Reflectance–Fourier Transform-Infrared Spectroscopy (ATR-FTIR)
2.5.2. Scanning Electron Microscopy (SEM)
2.5.3. Particle Size
2.6. Probiotic Cell Viability under INFOGEST Simulated Gastrointestinal Model
2.7. Statistical Analysis
3. Results and Discussion
3.1. Formulation of Wall Materials: Mixture Design
3.2. Characterization of Beads
3.2.1. Attenuated Total Reflectance–Fourier Transform-Infrared Spectroscopy (ATR-FTIR)
3.2.2. Particle Size and Morphological Characterization
3.3. Probiotic Cell Viability under INFOGEST-Simulated Gastrointestinal Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AlaAlam, M.S.; Aslam, R. Extrusion for the Production of Functional Foods and Ingredients. In Innovative Food Processing Technologies; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Helkar, P.B.; Sahoo, A.; Patil, N. Review: Food Industry By-Products used as a Functional Food Ingredients. Int. J. Waste Resour. 2016, 6, 1–6. [Google Scholar] [CrossRef]
- Reque, P.M.; Brandelli, A. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Haffner, F.B.; Diab, R.; Pasc, A. Encapsulation of probiotics: Insights into academic and industrial approaches. AIMS Mater. Sci. 2016, 3, 114–136. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Abid, M.B.; Koh, C.J. Probiotics in health and disease: Fooling Mother Nature? Infection 2019, 47, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Bultosa, G. Functional Foods: Dietary Fibers, Prebiotics, Probiotics, and Synbiotics. In Encyclopedia of Food Grains, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 2–4. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, S.; Fernández-Pacheco, P.; Seseña, S.; Pintado, C.; Palop, M.L. Selection of probiotic Lactobacillus strains with antimicrobial activity to be used as biocontrol agents in food industry. LWT 2021, 143, 111142. [Google Scholar] [CrossRef]
- Cueto, C.; Aragón, S. Evaluation of probiotic potential of lactic acid bacteria to reduce in vitro cholesterol. Sci. Agropecu. 2012, 1, 45–50. [Google Scholar] [CrossRef]
- Qi, X.; Simsek, S.; Chen, B.; Rao, J. International Journal of Biological Macromolecules Alginate-based double-network hydrogel improves the viability of encapsulated probiotics during simulated sequential gastrointestinal digestion: Effect of biopolymer type and concentrations. Int. J. Biol. Macromol. 2020, 165, 1675–1685. [Google Scholar] [CrossRef]
- Bauer-Estrada, K.; Sandoval-Cuellar, C.; Rojas-Muñoz, Y.; Quintanilla-Carvajal, M.X. The modulatory effect of encapsulated bioactives and probiotics on gut microbiota: Improving health status through functional food. Food Funct. 2022, 14, 32–55. [Google Scholar] [CrossRef]
- Kim, J.; Muhammad, N.; Jhun, B.H.; Yoo, J.-W. Probiotic delivery systems: A brief overview. J. Pharm. Investig. 2016, 46, 377–386. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Mishra, H.N. Novel approaches for co-encapsulation of probiotic bacteria with bioactive compounds, their health benefits and functional food product development: A review. Trends Food Sci. Technol. 2021, 109, 340–351. [Google Scholar] [CrossRef]
- Martín, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Microencapsulation of bacteria: A review of different technologies and their impact on the probiotic effects. Innov. Food Sci. Emerg. Technol. 2015, 27, 15–25. [Google Scholar] [CrossRef]
- Rodrigues, F.J.; Cedran, M.F.; Bicas, J.L.; Sato, H.H. Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications—A narrative review. Food Res. Int. 2020, 137, 109682. [Google Scholar] [CrossRef] [PubMed]
- Krasaekoopt, W.; Watcharapoka, S. Effect of addition of inulin and galactooligosaccharide on the survival of microencapsulated probiotics in alginate beads coated with chitosan in simulated digestive system, yogurt and fruit juice. LWT-Food Sci. Technol. 2014, 57, 761–766. [Google Scholar] [CrossRef]
- Lee, Y.; Ji, Y.R.; Lee, S.; Choi, M.J.; Cho, Y. Microencapsulation of probiotic lactobacillus acidophilus kbl409 by extrusion technology to enhance survival under simulated intestinal and freeze-drying conditions. J. Microbiol. Biotechnol. 2019, 29, 721–730. [Google Scholar] [CrossRef]
- Posbeyikian, A.; Tubert, E.; Bacigalupe, A.; Escobar, M.M.; Santagapita, P.R.; Amodeo, G.; Pellurini, M. Evaluation of calcium alginate bead formation kinetics: An integrated analysis through light microscopy, rheology and microstructural SAXS. Carbohydr. Polym. J. 2021, 269, 118293. [Google Scholar] [CrossRef] [PubMed]
- Brahma, S.; Sadiq, M.B.; Ahmad, I. Probiotics in Functional Foods; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780081005965. [Google Scholar]
- Pedroso-Santana, S.; Fleitas-Salazar, N. Ionotropic gelation method in the synthesis of nanoparticles/microparticles for biomedical purposes. Polym. Int. 2020, 69, 443–447. [Google Scholar] [CrossRef]
- Zazzali, I.; Rocio, T.; Calvo, A.; Manuel, V.; Ruíz-henestrosa, P.; Santagapita, P.R.; Perullini, M. E ff ects of pH, extrusion tip size and storage protocol on the structural properties of Ca(II)-alginate beads. Carbohydr. Polym. 2019, 206, 749–756. [Google Scholar] [CrossRef]
- Silva, M.P.; Tulini, F.L.; Martins, E.; Penning, M.; Fávaro-Trindade, C.S.; Poncelet, D. Comparison of extrusion and co-extrusion encapsulation techniques to protect Lactobacillus acidophilus LA3 in simulated gastrointestinal fluids. LWT-Food Sci. Technol. 2018, 89, 392–399. [Google Scholar] [CrossRef]
- Olivares, A.; Silva, P.; Altamirano, C. Microencapsulation of probiotics by efficient vibration technology. J. Microencapsul. 2017, 34, 667–674. [Google Scholar] [CrossRef]
- Benucci, I.; Cerreti, M.; Maresca, D.; Mauriello, G.; Esti, M. Yeast cells in double layer calcium alginate–chitosan microcapsules for sparkling wine production. Food Chem. 2019, 300, 125174. [Google Scholar] [CrossRef]
- Aragón-Rojas, S.; Quintanilla-Carvajal, M.X.; Hernández-Sánchez, H. Multifunctional Role of the Whey Culture Medium in the Spray-Drying Microencapsulation of Lactic Acid Bacteria. Food Technol. Biotechnol. 2018, 56, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Ricaurte, L.; Santagapita, P.R.; Díaz, L.E.; Quintanilla-Carvajal, M.X. Edible gelatin-based nanofibres loaded with oil encapsulating high-oleic palm oil emulsions. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 595, 124673. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Campaniello, D.; Speranza, B.; Racioppo, A.; Altieri, C.; Sinigaglia, M.; Corbo, M.R. Microencapsulation of Saccharomyces cerevisiae into Alginate Beads: A Focus on Functional Properties of Released Cells. Foods 2020, 9, 1051. [Google Scholar] [CrossRef] [PubMed]
- Graff, S.; Hussain, S.; Chaumeil, J.C.; Charrueau, C. Increased intestinal delivery of viable Saccharomyces boulardii by encapsulation in microspheres. Pharm. Res. 2008, 25, 1290–1296. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Labuschagne, P. Impact of wall material physicochemical characteristics on the stability of encapsulated phytochemicals: A review. Food Res. Int. 2018, 107, 227–247. [Google Scholar] [CrossRef]
- Yoha, K.S.; Nida, S.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Targeted Delivery of Probiotics: Perspectives on Research and Commercialization. Probiotics Antimicrob. Proteins 2021, 14, 15–48. [Google Scholar] [CrossRef]
- Chew, S.; Nyam, K. Microencapsulation of kenaf seed oil by co-extrusion technology. J. Food Eng. 2016, 175, 43–50. [Google Scholar] [CrossRef]
- Aguirre-Calvo, T.R.; Aguirre-Calvo, D.; Perullini, M.; Santagapita, P.R. A detailed microstructural and multiple responses analysis through blocking design to produce Ca(II)-alginate beads loaded with bioactive compounds extracted from by-products. Food Hydrocoll. Health 2021, 1, 100030. [Google Scholar] [CrossRef]
- Głąb, T.K.; Boratyński, J. Potential of Casein as a Carrier for Biologically Active Agents. Top. Curr. Chem. 2017, 375, 71. [Google Scholar] [CrossRef] [PubMed]
- Hébrard, G.; Hoffart, V.; Beyssac, E.; Cardot, J.M.; Alric, M.; Subirade, M. Coated whey protein/alginate microparticles as oral controlled delivery systems for probiotic yeast. J. Microencapsul. 2010, 27, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, S.S.; Alemzadeh, I.; Vaziri, A.S.; Vossoughi, A. Optimization of Alginate-Whey Protein Isolate Microcapsules for Survivability and Release Behavior of Probiotic Bacteria. Appl. Biochem. Biotechnol. 2020, 190, 182–196. [Google Scholar] [CrossRef]
- Donthidi, A.R.; Tester, R.F.; Aidoo, K.E. Effect of lecithin and starch on alginate-encapsulated probiotic bacteria. J. Microencapsul. 2010, 27, 67–77. [Google Scholar] [CrossRef]
- Shi, L.; Li, Z.; Li, D.; Xu, M.; Chen, H.; Zhang, Z.; Tang, Z. Encapsulation of probiotic Lactobacillus bulgaricus in alginate—Milk microspheres and evaluation of the survival in simulated gastrointestinal conditions. J. Food Eng. 2013, 117, 99–104. [Google Scholar] [CrossRef]
- Li, X.Y.; Chen, X.G.; Cha, D.S.; Park, H.J.; Liu, C.S. Microencapsulation of a probiotic bacteria with alginategelatin and its properties. J. Microencapsul. 2009, 26, 315–324. [Google Scholar] [CrossRef]
- Khalil, K.A.; Mustafa, S.; Mohammad, R.; Bin Ariff, A.; Ahmad, S.A.; Dahalan, F.A.; Manap, M.Y.A. Encapsulation of Bifidobacterium pseudocatenulatum Strain G4 within Bovine Gelatin-Genipin-Sodium Alginate Combinations: Optimisation Approach Using Face Central Composition Design-Response Surface Methodology (FCCD-RSM). Int. J. Microbiol. 2019, 2019, 4208986. [Google Scholar] [CrossRef]
- Aguirre-Calvo, T.R.; Molino, S.; Perullini, M.; Rufián-Henares, J.Á.; Santagapita, P.R. Effect of in vitro digestion-fermentation of Ca(II)-alginate beads containing sugar and biopolymers over global antioxidant response and short chain fatty acids production. Food Chem. 2020, 333, 127483. [Google Scholar] [CrossRef]
- Santagapita, P.R.; Mazzobre, M.F.; Buera, P. Invertase stability in alginate beads Effect of trehalose and chitosan inclusion and of drying methods. FRIN 2012, 47, 321–330. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Ramos, M.; Gómez-Ruiz, J.Á. Bioactive components of ovine and caprine cheese whey. Small Rumin. Res. 2011, 101, 196–204. [Google Scholar] [CrossRef]
- Corfield, R.; Martínez, K.D.; Allievi, M.C.; Santagapita, P.; Mazzobre, F.; Schebor, C.; Pérez, O.E. Whey proteins-folic acid complexes: Formation, isolation and bioavailability in a Lactobacillus casei model. Food Struct. 2020, 26, 100162. [Google Scholar] [CrossRef]
- Smidsrød, O.; Larsen, B.; Painter, T.; Haug, A.R. The role of intramolecular autocatalysis in the acid hydrolysis of polysaccharides containing 1,4-linked hexuronic acid. Acta Chem. Scand. 1969, 23, 1573–1580. [Google Scholar] [CrossRef]
- Wolkers, W.F.; Oliver, A.E.; Tablin, F.; Crowe, J.H. A Fourier-transform infrared spectroscopy study of sugar glasses. Carbohydr. Res. 2004, 339, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, P.H.P.; Charalampopoulos, D. Food Bioscience Encapsulation of Bi fi dobacterium longum in alginate-dairy matrices and survival in simulated gastrointestinal conditions, refrigeration, cow milk and goat milk. Food Biosci. 2018, 21, 72–79. [Google Scholar] [CrossRef]
- Lopes, S.; Bueno, L.; Júnior, F.D.A.; Finkler, C. Preparation and characterization of alginate and gelatin microcapsules containing Lactobacillus rhamnosus. An. Acad. Bras. Cienc. 2017, 89, 1601–1613. [Google Scholar] [CrossRef]
- Parker, E.A.; Roy, T.; D’Adamo, C.R.; Wieland, L.S. Probiotics and gastrointestinal conditions: An overview of evidence from the Cochrane Collaboration. Nutrition 2018, 45, 125–134.e11. [Google Scholar] [CrossRef]
- Beldarrain Iznaga, T.; Villalobos Carvajal, R.; Leiva Vega, J.; Sevillano Armesto, E. Food and Bioproducts Processing Influence of multilayer microencapsulation on the viability of Lactobacillus casei using a combined double emulsion and ionic gelation approach. Food Bioprod. Process. 2020, 124, 57–71. [Google Scholar] [CrossRef]
- De Prisco, A.; van Valenberg, H.J.F.; Fogliano, V.; Mauriello, G. Microencapsulated Starter Culture During Yoghurt Manufacturing, Effect on Technological Features. Food Bioprocess Technol. 2017, 10, 1767–1777. [Google Scholar] [CrossRef]
- Ramos, P.E.; Abrunhosa, L.; Pinheiro, A.; Cerqueira, M.A.; Motta, C.; Castanheira, I.; Chandra-Hioe, M.V.; Arcot, J.; Teixeira, J.A.; Vicente, A.A. Probiotic-loaded microcapsule system for human in situ folate production: Encapsulation and system validation. Food Res. Int. 2016, 90, 25–32. [Google Scholar] [CrossRef]
- Ta, L.P.; Bujna, E.; Antal, O.; Ladányi, M.; Juhász, R.; Szécsi, A.; Kun, S.; Sudheer, S.; Gupta, V.K.; Nguyen, Q.D. Effects of various polysaccharides (alginate, carrageenan, gums, chitosan) and their combination with prebiotic saccharides (resistant starch, lactosucrose, lactulose) on the encapsulation of probiotic bacteria Lactobacillus casei 01 strain. Int. J. Biol. Macromol. 2021, 183, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Eckert, C.; Agnol, W.D.; Dallé, D.; Serpa, V.G.; Maciel, M.J.; Lehn, D.N.; Volken de Souza, C.F. Development of alginate-pectin microparticles with dairy whey using vibration technology: Effects of matrix composition on the protection of Lactobacillus spp. from adverse conditions. Food Res. Int. 2018, 113, 65–73. [Google Scholar] [CrossRef] [PubMed]




| Run | Factors | Response Variable | ||
|---|---|---|---|---|
| A: Alginate | B: Gelatin | C: Sweet Whey | Viability (log10 CFU/mL) | |
| 1 | 0.80 | 0.02 | 0.18 | 9.12 |
| 2 | 0.50 | 0.26 | 0.24 | 9.05 |
| 3 | 0.55 | 0.00 | 0.45 | 9.11 |
| 4 | 0.40 | 0.10 | 0.50 | 8.91 |
| 5 | 0.66 | 0.34 | 0.00 | 9.14 |
| 6 | 0.90 | 0.06 | 0.04 | 8.47 |
| 7 | 0.50 | 0.26 | 0.24 | 8.95 |
| 8 | 0.52 | 0.37 | 0.11 | 9.18 |
| 9 | 0.66 | 0.34 | 0.00 | 9.04 |
| 10 | 0.40 | 0.50 | 0.10 | 8.38 |
| 11 | 0.65 | 0.15 | 0.20 | 9.05 |
| 12 | 0.40 | 0.10 | 0.50 | 8.91 |
| 13 | 0.76 | 0.18 | 0.06 | 8.87 |
| 14 | 0.50 | 0.26 | 0.24 | 9.04 |
| 15 | 0.68 | 0.00 | 0.32 | 9.26 |
| 16 | 0.40 | 0.50 | 0.10 | 8.65 |
| Sum of Squares | Degrees of Freedom | p-Value | |
|---|---|---|---|
| Model | 0.751 | 5 | 0.003 |
| Linear | 0.126 | 2 | 0.078 |
| AB | 0.491 | 1 | 0.005 |
| AC | 0.359 | 1 | 0.001 |
| BC | 0.001 | 1 | 0.834 |
| Residuals | 0.190 | 10 | |
| Lack of fit | 0.149 | 6 | 0.208 |
| Pure Error | 0.041 | 4 | |
| R2 | 0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Muñoz, Y.V.; Santagapita, P.R.; Quintanilla-Carvajal, M.X. Probiotic Encapsulation: Bead Design Improves Bacterial Performance during In Vitro Digestion. Polymers 2023, 15, 4296. https://doi.org/10.3390/polym15214296
Rojas-Muñoz YV, Santagapita PR, Quintanilla-Carvajal MX. Probiotic Encapsulation: Bead Design Improves Bacterial Performance during In Vitro Digestion. Polymers. 2023; 15(21):4296. https://doi.org/10.3390/polym15214296
Chicago/Turabian StyleRojas-Muñoz, Yesica Vanesa, Patricio Román Santagapita, and María Ximena Quintanilla-Carvajal. 2023. "Probiotic Encapsulation: Bead Design Improves Bacterial Performance during In Vitro Digestion" Polymers 15, no. 21: 4296. https://doi.org/10.3390/polym15214296
APA StyleRojas-Muñoz, Y. V., Santagapita, P. R., & Quintanilla-Carvajal, M. X. (2023). Probiotic Encapsulation: Bead Design Improves Bacterial Performance during In Vitro Digestion. Polymers, 15(21), 4296. https://doi.org/10.3390/polym15214296