3.1. Characterization of Ti-APP and Ti-CaCO3
Thermogravimetric analysis characterizes the mass change in the material during the temperature-programmed process. It reflects the sensitivity and stability of the material to heat [
36]. Materials with higher initial decomposition temperature and carbon residue rate tend to have better flame-retardant properties. (
Figure 5) and (
Table 2) are the thermogravimetric test curves and characteristic parameter tables of modified APP and modified CaCO
3, respectively. It can be found from
Figure 4 that the modified APP and CaCO
3 are significantly different compared with the unmodified ones. The characteristic data in
Table 2 can more intuitively show the difference before and after modification. The modified APP (Ti-APP) still decomposes twice during the temperature programmed process, which is consistent with the decomposition process of unmodified APP. However, the heat resistance of Ti-APP was improved after modification, and its initial decomposition temperature (
T-5%,
T-10%) and the first fastest decomposition temperature (
Tmax) were increased by about 10 °C, respectively. The fastest decomposition temperature for the second time even increased by 25 °C. Unfortunately, the residual carbon rate of Ti-APP is lower than that of APP.
After modification, Ti-CaCO3 turned to decompose in two steps similar to APP during the temperature programmed process of 25–800 °C, and its heat resistance also changed obviously. The initial decomposition temperature (T-10%) was advanced by 21 °C which may be caused by the decomposition of the titanate coupling agent coated on its surface, but the fastest decomposition temperature (Tmax) in the second step was delayed by 21 °C indicating that the decomposition process of Ti-CaCO3 is more gradual, and the addition of titanate slows down its decomposition process.
The infrared spectra of APP, CaCO
3, and their modification are shown in (
Figure 6). PN-201 mainly has two characteristic peaks. From 1030 cm
−1 to 740 cm
−1, the infrared absorption decreases continuously and shows strong absorption corresponding to Ti-O. Additionally, the antisymmetric vibration peak of C-H appears around 2934 cm
−1 [
37]. In
Figure 5, Ti-APP exhibits the characteristic absorption peak of Ti-O at 1015 cm
−1, while Ti-CaCO
3 shows it at 1064 cm
−1. The absorption of Ti-CaCO
3 at 2929 cm
−1 is attributed to the C-H antisymmetric vibration peak. Comparatively speaking, the C-H absorption of Ti-APP appears at 3054 cm
−1 showing a blue shift, which may be due to the shift of the absorption peak to the high wave number caused by the induction effect. The characteristic peak of PN-201 was detected in the modified APP and CaCO
3, indicating that it was successfully loaded on APP and CaCO
3.
The XRD spectra of APP, CaCO
3, and their modification are shown in (
Figure 7). The characteristic diffraction peaks of CaCO3 and Ti-CaCO3 were found at 23°, 29°, 36°, 39°, 43°, 47.5°, 48.5° [
38]. Both APP and Ti-APP found their characteristic diffraction peaks at 16.7°, 15.5°, 20°, 22°, 26°, 27.6°, 29°, 30.6°, 35.7°, 36.5°, 38° [
39]. According to it, the spectra of Ti-APP and Ti-CaCO
3 after modification have no obvious change from those before modification. This indicates that the modification of APP and CaCO3 by PN-201 does not change their crystal morphology and has no obvious effect on their structure and state.
3.2. Flammability of FR-POM Composites
UL-94 and LOI tests are often used to initially characterize the combustion properties of materials. According to the analysis of them, the char formation of the intumescent material during the combustion process, and the compactness of the char formation can be identified [
40]. The test results of UL-94 and LOI are shown in (
Table 3).
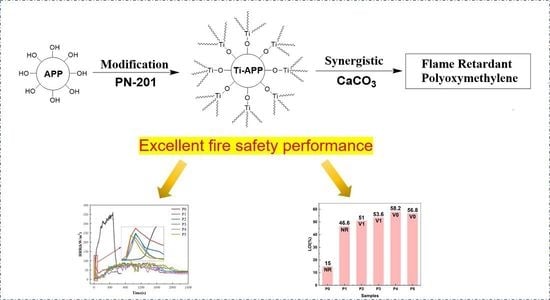
Pure POM has virtually no flame retardancy. Once ignited, it will burn violently and produce combustion dripping, so it fails to reach the flame-retardant level and its LOI value only reaches 15%. After IFR is added, the combustion drip–drop phenomenon disappears, and the LOI of P1 reaches 46.6%, which is more than three times that of P0, indicating that P1 has good carbon formation performance in the combustion process. However, UL-94 results showed that P1’s second ignition time was too long to reach flame retardant level. It indicates that the carbon layer cannot effectively block the release of combustible gas inside the material, and the density is still insufficient. After APP was modified by PN-201, the flame-retardant performance was further improved. The P2 achieves a V1 rating with an improved LOI value of 51%. The synergistic effect of adding CaCO3 to the IFR makes P3 reach the UL-94 V1 level, and the LOI value is increased to 53.6%. It is shown that whether the modified APP (Ti-APP) or the synergistic effect of CaCO3 is used, the carbon formation of FR-POM composites can be further enhanced, and the compactness of the carbon layer can be significantly improved.
IFR with Ti-APP as acid source (Ti-IFR) synergistic CaCO3 achieved the most excellent synergistic flame-retardant effect among all the FR-POM. The P4 achieves a UL-94 V0 rating and a high LOI value of 58.2%, far better than P2, P3, and even P5. The performance of P5 with modify CaCO3 (Ti-CaCO3) and Ti-IFR is only inferior to that of P4. It also reaches the UL-94 V0 level, but its LOI of 56.8% is slightly lower than P4. It may be due to the decrease in thermal stability of Ti-CaCO3 leading to a slight decrease in carbon formation and carbon layer compactness of P5, compared with unmodified CaCO3.
3.3. Thermogravimetric Analysis
The TG and DTG curves of FR-POM composites under N
2 and air atmospheres are shown in (
Figure 8), and the thermogravimetric data are summarized in (
Table 4 and
Table 5). It can be seen from
Figure 7 that the decomposition trends of FR-POM composites under N
2 and air atmospheres are almost the same, both showing one-step decomposition. However, the N
2 atmosphere seems to be more conducive to the formation of carbon residues, which may be due to the participation of oxygen in the air atmosphere to generate CO
2, thereby reducing the amount of carbon residues [
41]. In both atmospheres, the initial decomposition temperature
T-5% of P0 is around 233 °C,
T-10% is around 248 °C, and the fastest decomposition temperature is about 295 °C. Moreover, the whole decomposition process of P0 was smoother than others, the decomposition was more thorough, and there was basically no carbon residue left. After adding different flame retardants, the thermal decomposition of FR-POM is very similar. The initial decomposition temperature
T-5% is increased by about 20 °C compared with P0, but
Tmax is only about 260 °C, which is very close to
T-10% and
T-5%. The thermal resistance of P1, P2, P3, P4, and P5 is improved, and the thermal decomposition temperature range is narrow. It may be that the addition of flame retardants not only improves the heat resistance of composite materials but also disturbs the original structured molecular structure of POM so that its decomposition process is very rapid and shows a narrow decomposition range.
The carbon residue rate at 800 °C reflects the carbon-forming ability of the materials. In the N2 atmosphere, the addition of IFR makes the carbon residue rate of P1 reach 15.2%. After using Ti-IFR, the carbon residue rate of P2 is further improved, reaching 17.1%. While the carbon residue rate of P3 with CaCO3 as a synergist is only 14.0% indicating that Ti-APP can improve the carbon residue rate more efficiently than CaCO3. Unexpectedly, the synergistic system of CaCO3 and Ti-IFR achieved the best carbon formation effect. The carbon residue rate of P4 reached 18.2%, which was 19.7% higher than that of P1. However, by combining Ti-CaCO3 with Ti-IFR, the carbon residue rate of P5 is slightly lower than that of P4. It shows that Ti-IFR and CaCO3 have an excellent synergistic flame-retardant effect and excellent carbon-forming properties. In addition, the FR-POM composites also showed similar carbon-forming ability in the air atmosphere. Furthermore, the carbon residue rate of P3, P4, and P5 with calcium carbonate in the AIR atmosphere is apparently higher than that of P1 and P2 without it, indicating that CaCO3, whether modified or not, is highly efficient to promote the carbon-forming and synergistic effect with IFR under air atmosphere.
3.4. Cone Calorimeter Research
Cone calorimetry (CONE) is a method to record information about the combustion state of materials in a simulated real fire environment. As a very reliable test, it has been widely used in the evaluation of flame-retardant properties of composite materials such as various engineering plastics, general plastics, foam materials, and wood materials [
38]. Characteristic data such as time to ignition (TTI), heat release rate (HRR), total heat release (THR), effective heat of combustion (EHC), total smoke generation (TSP), and specific extinction area (SEA) can be obtained. Through these data, an objective analysis of the material’s heat release, gas release, and flame-retardant mechanism can be carried out. The curves of HRR, THR, TSP, and mass loss rate (MLR) of FR-POM composites as a function of time are shown in (
Figure 9). The characteristic data obtained from the cone calorimetry test are listed in (
Table 6).
As shown in
Figure 8, P0 was ignited at 43 s, the peak heat release rate (Pk HRR) reached 361.2 kW/m
2, and the THR amounted to 140.7 MJ/m
2. The HRR curve peaks in a very short time, so the average heat release rate (Av HRR) is as high as 267.6 kW/m
2. After the introduction of flame-retardant additives, the ignition time of all FR-POM was significantly earlier than that of P0, probably due to the decomposition of IFR at high temperatures to perform its flame-retardant effect and lead to the earlier decomposition time of FR-POM composites.
The flame-retardant effect of IFR is very effective and significant. The Pk HRR value of P1 is only 124.6 kW/m2, and the THR value is only 107.9 kW/m2. Compared with P0, the Pk HRR and THR of P1 are decreased by 65.5% and 23.3%, respectively. In addition, because of the prolonged burning time, the Av HRR value of P1 decreased by 77.6%. The HRR value characterizes the heat release rate per second of the material and is closely related to the total heat released (THR) during the combustion process. Obviously, the higher the HRR and THR of the material, the higher the fire risk.
After using Ti-IFR instead of general IFR, the Pk HRR and the mean effective heat of combustion (mean EHC) of P2 decreased by 16.3% and 8.1%, respectively, compared with that of P1, but both Av HRR and THR increased slightly. Moreover, the specific extinction area (SEA) increased by 81.4%, the total smoke emission (TSP) of P2 is 2.1 times that of P1, while the amount of carbon residue is 0.8 times of P1, which fully shows that Ti-IFR plays a flame-retardant role more in the gas phase than IFR.
Compared with P1, the Pk HRR of P3 decreased by 19.5%, and its Av HRR and THR also decreased a little. Meanwhile, the mean EHC was basically unchanged, and SEA increased slightly. In addition, the amount of residual carbon of P3 is two times that of P1, but the total smoke emission is almost the same. It is proved that the flame-retardant effect was exerted mainly in the condensed phase. It is the large amount of carbon residue that effectively isolates the contact between the combustible gas inside the matrix, oxygen, and the external heat so that the combustion process tends to stagnate.
P2 and P3 show different flame-retardant mechanisms. The former with Ti-IFR further strengthens the gas phase flame retardant effect of IFR, while the latter with CaCO3 further improves the condensed phase flame retardant effect. In order to enhance both gas phase and condensed phase performance at the same time, Ti-IFR was compounded with CaCO3 to flame retardant POM. As a result, the Pk HRR, Av HRR and THR values of P4 decreased by 75.8%, 81.1%, and 35.3% compared to pure POM, respectively. The flame-retardant effect is far better than P1, P2, and P3. Its mean EHC was slightly decreased, and the SEA was increased compared with P3, indicating a gas phase flame retardant mechanism. Moreover, compared with P2, the mean EHC of P4 was basically unchanged, the SEA and TSP were significantly reduced, and the amount of carbon residue was increased by 2.6 times, which is an obvious condensed phase flame retardant mechanism. Therefore, P4 achieves an excellent effect by combining two flame retardant mechanisms that enhance simultaneously.
Like P4, P5 has excellent flame retardancy which uses PN-201 modified CaCO3 (Ti-CaCO3) and Ti-IFR to synergize flame retardant POM. Its Pk HRR, Av HRR, and THR decreased by 73.3%, 75.4%, and 16% compared with pure POM, respectively. However, its flame-retardant properties are slightly lower than P4, although it is better than other samples. The amount of residual carbon was significantly reduced, and the mean EHC was increased indicating that the condensed phase flame retardancy is restrained after loading Ti-CaCO3. It may be because of the addition of Ti-CaCO3 that the content of PN-201 in the system increased slightly as a whole breaking the original equilibrium ratio of the gas phase and condensed phase, resulting in a decrease in synergy.
3.5. Carbon Layer Analysis of FR-POM Composites
In order to further analyze the integrity and strength of the carbon layer, the surface carbon layer of the FR-POM composite after cone calorimetry was analyzed by digital photographs, field emission scanning electron microscopy (SEM) and laser Raman spectroscopy (LRS) [
42,
43,
44]. Digital photos, SEM pictures and LRS spectra are shown in (
Figure 10,
Figure 11 and
Figure 12) respectively.
Composites with IFR generate large amounts of gas during combustion due to the presence of blowing agents. If the carbon layer has good compactness and high strength, it is difficult for all the gases to break through and will inevitably expand. Therefore, the expansion height of the residual carbon can often preliminarily judge the strength and compactness of the carbon layer, as well as the flame-retardant performance [
45]. As shown in the digital photo of carbon residue in
Figure 10, pure POM has almost no carbon residue. After adding flame retardant filler, the carbon residue increases significantly, especially for the sample added with calcium carbonate. Ti-IFR furthers the flame-retardant performance in the gas phase, so the carbon layer of P2 is a little fragmented with no obvious difference from that of P1. While P3, P4, and P5 added with CaCO
3 mainly act in the condensed phase, their carbon layers are obviously denser and higher than P1. P4 and P5 have the best flame-retardant properties in CONE due to the synergistic effect which is also reflected in digital photos in terms of carbon layer strength, swelling height, and compactness.
The digital photos of carbon residues are used to preliminarily judge the strength and compactness of the carbon layer from the macroscopic point of view, while the SEM photos are observed from the microscopic point of view. In the observed field of SEM image as shown in
Figure 11, there are many pores in the carbon layer of P1, accompanied by a few cracks. P2 shows a fragmented carbon layer like P1, but basically has no holes and still has a certain degree of compactness. It may be related to its gas phase flame retardant mechanism. The carbon layer of P3 is much denser than that mentioned above, with very few cracks and sporadic holes in the field of view. This is closely related to its condensed phase flame retardant mechanism. In the field of view of P4, there are basically no holes and cracks, and the surface of the carbon layer is smooth and flat, which is consistent with the results of its flame-retardant properties. The carbon layer of P5 has a few holes, but basically no cracks.
The LRS of the carbon layer mainly shows two peak positions. As shown in
Figure 12, one is around 1350 cm
−1 (D peak), which represents lattice defects in the disordered carbon structure. The other is around 1580 cm
−1 (G peak), representing the sp
2 hybridization of the ordered carbon structure with in-plane stretching vibrations. The graphitization degree of the carbon layer is usually indicated by the peak area ratio (I
D/I
G) of the D peak to the G peak. Obviously, the smaller the I
D/I
G value, the higher the degree of graphitization of the carbon layer, which is more beneficial for the surface carbon layer to resist flame burning [
46]. The I
D/I
G values of P1 and P2 are 0.72 and 0.75 with little difference indicating that Ti-IFR has no contribution to the degree of graphitization of the carbon layer. While the I
D/I
G values of P3, P4, and P5 are significantly lower than that of P1 and P2. This demonstrates that CaCO
3, whether modified or not, can improve the degree of graphitization of the carbon layer. Especially, P4 has the highest degree of graphitization with the smallest I
D/I
G value of 0.57 among all the samples, which is consistent with its flame-retardant performance.
From the FTIR spectrum of the carbon layer (
Figure 13), it can be clearly found that the residual carbon spectra mainly include the absorption peak of OH (3431 cm
−1), C=O (1630 cm
−1), P-O (1184 cm
−1) and P=O symmetric stretching vibration (985 cm
−1). P0 has obvious -OH and C=O peaks at 3431 cm
−1 and 1630 cm
−1. As to the FR-POM samples, their FTIR spectrum is very similar. APP in the additives can produce metaphosphoric acid and pyrophosphoric acid compounds at high temperatures, which catalyzed the ring-opening polymerization of the BOZ carbon agent but also promoted the dehydration and carbonization of the matrix. Thus, significantly weakened the -OH and C=O peaks of P1-P5, and enhanced the absorption peaks of P-O and P=O. After adding CaCO
3, the peak at around 1184 cm
−1 of P3–P5 decreased obviously and became a broad one, which is different from that of P1 and P2. It indicated that CaCO
3 participates in the formation of the carbon layer and changes the mechanism of carbon formation. The reason may be due to the degradation of CaCO
3 to CaO at high temperatures, which acts as a strong alkaline catalyst to promote the esterification and cross-linking reaction resulting in a high-quality carbon layer.
The EDS data in (
Table 7) reveal the main components of the expanded carbon layer. The stable and dense expanded carbon layer is mainly formed by the elements of C, N, O, P, and Ca. The carbon-to-oxygen ratios (C/O) of the carbon layer on the surface of P1-5 are all around 0.5 and greatly lower than their corresponding inner carbon layer. This is because the carbon layer on the surface burns more fully in contact with the flame with a higher oxidation degree to form a more heat-resistant and high-temperature-resistant oxide to protect the inner carbon layer from being burned. The change in the C/O ratio of the inner carbon layer of all samples strongly proves this point. P4 has the best flame-retardant performance, and its surface carbon layer has strong compactness. Therefore, the protection of the inner carbon layer is the best, the C/O of the inner carbon layer is 2.7, and the degree of oxidation is extremely low. In addition, no Ti element was detected in all samples, probably due to the fact that it mainly functions in the gas phase and is used in a very small amount.
3.6. Gaseous Products Analysis
The TG-IR combination method was used to obtain information on gas volatiles in FR-POM composite as a function of temperature. The 3D spectra of P0 and P4 are shown in (
Figure 14). Since the temperature range of the TG test is 25–800 °C, and the heating rate is 10 °C/min. Therefore, the relationship between time and temperature in
Figure 14 is:
(
Te refers to temperature and t
i refers to time).
It can be found from the 3D figure that the types of gas volatiles of P0 and P4 composites are basically the same in the nitrogen atmosphere. Mainly carbonyl compounds (CH2O, 1715 cm−1, and 1773 cm−1), aliphatic lipids (1745 cm−1), and hydrocarbon groups such as methyl and ethyl groups (2801–2924 cm−1). The results show that FR-POM composites crack mainly in the form of formaldehyde and various small molecular chain alkanes under the condition of high temperatures without oxygen. In addition, the absorption peaks of C-O (1161 cm−1), the symmetric stretching vibration of -CH3 (1320 cm−1), the skeleton stretching vibration of aromatic hydrocarbons (1458–1532 cm−1), and the absorption of N-H (3468 cm−1) can still be found in the 3D diagram.
In the air atmosphere, P0 and P4 composites have an obvious CO2 absorption peak (2358 cm−1), which may be due to the participation of oxygen, prompting a part of formaldehyde or small molecular chain alkanes to completely burn, thus producing CO2. Moreover, the CO2 absorption peak of P4 was significantly lower than that of P0 due to the addition of flame retardant. The results show that the addition of flame-retardant leads to incomplete combustion of the material and locks more C in the carbon layer, thus effectively reducing the corrosion of the gas volatiles. In addition, the decomposition is basically the same as that in the nitrogen atmosphere.
The FTIR spectra of FR-POM composites with the maximum release of gas residues are shown in (
Figure 15). It can be found that the decomposition of other groups is basically the same as that of P4. All FR-POM composites showed mainly flammable gas release such as aldehydes, hydrocarbons, and lipids, and no typical titanium-containing substances were found, mainly due to the very small proportion of PN-201 in the overall system.
3.7. Flame Retardant Mechanism
Combined with the previous analysis, we put forward the following possible flame-retardant process mechanism. As shown in (
Figure 16), when FR-POM composites are continuously exposed to fire or heat sources, the flame-retardant additives begin to take effect mainly in the condensed phase as well as in the gas phase simultaneously.
In the condensed phase, the high temperature makes the surface of the matrix dehydrate and carbonize rapidly, which has a certain protective effect on the interior of the matrix. With the continuous high-temperature erosion, the surface carbon layer will be destroyed, the internal matrix will be affected by the high temperature and the constant release of flammable gas. At this point, the BOZ carbonating agent comes into play. The pyrophosphoric acid and metaphosphate decomposed by APP catalyze the esterification and crosslinking of BOZ, and finally make it become a stable carbon layer with a three-dimensional network structure, which greatly improves the density and strength of the carbon layer, thus blocking the communication channel between the combustible gas in the matrix and the external heat source, and finally interrupts the combustion process [
41]. The addition of calcium carbonate further catalyzes BOZ and plays a stronger catalytic carbonization role in the matrix, thus accelerating the formation of the carbon layer.
In the gas phase, the matrix structure is destroyed, and a large number of formats and hydrocarbon-based combustible gases are released, which makes the heat release peak quickly. At the same time, when IFR flame retardant is decomposed by heat, ME quickly decomposes into refractory nitrogen and begins to dilute flammable gas in the gas phase with a large amount of carbon dioxide produced by matrix combustion, thus reducing the intensity of combustion. In addition, TiOxprodued by Ti-APP under high temperature scavenges the high activity free radicals such as H· and HO· bringing the combustion process to a standstill. Thus, a significant flame-retardant performance was achieved (i.e., the gas phase TiO clears the incomplete combustion caused).