Dispersion and Homogeneity of MgO and Ag Nanoparticles Mixed with Polymethylmethacrylate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Study Groups
NPs (MgO and Ag) Groups
- A.
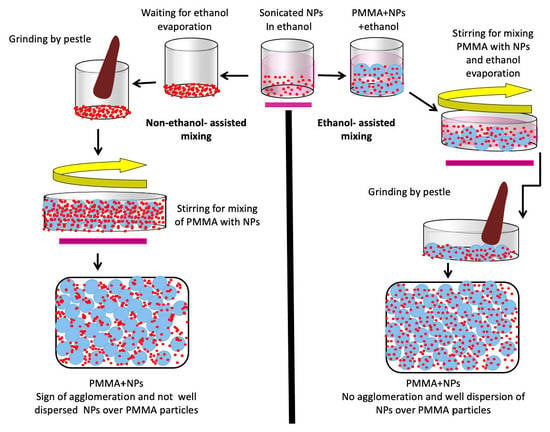
- Non-ethanol-assisted mixing of NPs with PMMA powder (without ethanol)
- B.
- Ethanol-assisted mixing of NP with PMMA powder (with ethanol)
Control Group
2.2.2. Discs Preparation
2.3. Dispersion and Homogeneity Tests
2.3.1. X-ray Diffraction (XRD)
2.3.2. Energy-Dispersive X-ray Spectroscopy (EDX)
2.3.3. Scanning Electron Microscope (SEM)
2.3.4. Stereo Microscope
3. Results
3.1. X-ray Diffraction Test
3.2. Energy-Dispersive X-ray Spectroscopy (EDX)
3.3. Scanning Electron Microscope (SEM)
3.4. Stereo Microscope Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elshereksi, N.W.; Ghazali, M.J.; Muchtar, A.; Azhari, C.H. Perspectives for Titanium-Derived Fillers Usage on Denture Base Composite Construction: A Review Article. Adv. Mater. Sci. Eng. 2014, 2014, 746252. [Google Scholar] [CrossRef] [Green Version]
- Brinson, H.F.; Brinson, L.C. Characteristics, applications and properties of polymers. In Polymer Engineering Science and Viscoelasticity; Springer: Cham, Switzerland, 2015; pp. 57–100. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Ebrahim, M.I. Effect of Zirconium Oxide Nano-Fillers Addition on the Flexural Strength, Fracture Toughness, and Hardness of Heat-Polymerized Acrylic Resin. World J. Nano Sci. Eng. 2014, 4, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Sakaguchi, R.L.; Powers, J.M. Craig’s Restorative Dental Materials E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Bistolfi, A.; Ferracini, R.; Albanese, C.; Vernè, E.; Miola, M. PMMA-Based Bone Cements and the Problem of Joint Arthroplasty Infections: Status and New Perspectives. Materials 2019, 12, 4002. [Google Scholar] [CrossRef] [Green Version]
- Radford, D.; Challacombe, S.J.; Walter, J.D. Denture Plaque and Adherence of Candida Albicans to Denture-Base Materials in Vivo and in Vitro. Crit. Rev. Oral Biol. Med. 1999, 10, 99–116. [Google Scholar] [CrossRef]
- Murakami, N.; Wakabayashi, N.; Matsushima, R.; Kishida, A.; Igarashi, Y. Effect of High-Pressure Polymerization on Mechanical Properties of PMMA Denture Base Resin. J. Mech. Behav. Biomed. Mater. 2013, 20, 98–104. [Google Scholar] [CrossRef]
- Yildirim, M.S.; Hasanreisoǧlu, U.; Hasirci, N.; Sultan, N. Adherence of Candida Albicans to Glow-discharge Modified Acrylic Denture Base Polymers. J. Oral Rehabil. 2005, 32, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Mittal, R.; Sood, V.K.; Garg, R. Effect of Incorporation of Silane-treated Silver and Aluminum Microparticles on Strength and Thermal Conductivity of PMMA. J. Prosthodont. Implant. Esthet. Reconstr. Dent. 2012, 21, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Torres, L.S.; Mendieta, I.; Nuñez-Anita, R.E.; Cajero-Juárez, M.; Castaño, V.M. Cytocompatible Antifungal Acrylic Resin Containing Silver Nanoparticles for Dentures. Int. J. Nanomed. 2012, 7, 4777–4786. [Google Scholar] [CrossRef] [Green Version]
- Akkuş, B.; Ozturk, A.N.; Yazman, Ş.; Akdemir, A. Effects of Al2O3 and SiO2 Nanoparticles on Flexural Strength of Heat Cured Acrylic Resin. Int. J. Enhan. Res. Sci. Technol. Eng. 2015, 4, 158–163. [Google Scholar]
- Adhikari, R.; Michler, G.H. Polymer Nanocomposites Characterization by Microscopy. J. Macromol. Sci. Part C Polym. Rev. 2009, 49, 141–180. [Google Scholar] [CrossRef]
- de Souza Neto, F.N.; Sala, R.L.; Fernandes, R.A.; Xavier, T.P.O.; Cruz, S.A.; Paranhos, C.M.; Monteiro, D.R.; Barbosa, D.B.; Delbem, A.C.B.; de Camargo, E.R. Effect of Synthetic Colloidal Nanoparticles in Acrylic Resin of Dental Use. Eur. Polym. J. 2019, 112, 531–538. [Google Scholar] [CrossRef]
- Aldabbagh, B.; Jawad, H.; Mahdi, R. Study of the Properties of MgO/Poly Methyl Methacrylate Nano-Composites. J. Phys. Conf. Ser. 2021, 2114, 012039. [Google Scholar] [CrossRef]
- Jabbarzadeh, A.; Halfina, B. Unravelling the Effects of Size, Volume Fraction and Shape of Nanoparticle Additives on Crystallization of Nanocomposite Polymers. Nanoscale Adv. 2019, 1, 4704–4721. [Google Scholar] [CrossRef] [Green Version]
- Balazs, A.C.; Emrick, T.; Russell, T.P. Nanoparticle Polymer Composites: Where Two Small Worlds Meet. Science 2006, 314, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhong, J. Effect of Dispersion of Nano-Inorganic Particles on the Properties of Polymer Nanocomposites. IOP Conf. Ser. Mater. Sci. Eng. 2019, 563, 022026. [Google Scholar] [CrossRef]
- Liu, H.; Webster, T.J. Mechanical Properties of Dispersed Ceramic Nanoparticles in Polymer Composites for Orthopedic Applications. Int. J. Nanomed. 2010, 2010, 299–313. [Google Scholar] [CrossRef] [Green Version]
- Ashour, M.; El-Shennawy, M.; Omar, A.A.; Ebrahim, M.I.; Althomali, Y. Influence of Addition of Different Types of Nano-Fillers on the Microstructure and Mechanical Properties of PMMA Based Denture Resin. Kasmera 2017, 45, 48–59. [Google Scholar]
- Sun, J.; Wang, L.; Wang, J.; Li, Y.; Zhou, X.; Guo, X.; Zhang, T.; Guo, H. Characterization and Evaluation of a Novel Silver Nanoparticles-Loaded Polymethyl Methacrylate Denture Base: In Vitro and in Vivo Animal Study. Dent. Mater. J. 2021, 40, 1100–1108. [Google Scholar] [CrossRef]
- Gad, M.M.; Abualsaud, R.; Alqarawi, F.K.; Emam, A.N.M.; Khan, S.Q.; Akhtar, S.; Mahrous, A.A.; Al-Harbi, F.A. Translucency of Nanoparticle-Reinforced Pmma Denture Base Material: An in-Vitro Comparative Study. Dent. Mater. J. 2021, 40, 972–978. [Google Scholar] [CrossRef]
- Boulerba, D.; Zoukel, A. Nanocomposites: Effects of the Molecular Interaction Strength on Thermal Properties. Polym. Polym. Compos. 2021, 29, S49–S56. [Google Scholar]
- Yeap, S.P. Permanent Agglomerates in Powdered Nanoparticles: Formation and Future Prospects. Powder Technol. 2018, 323, 51–59. [Google Scholar] [CrossRef]
- Estrada-Monje, A.; Zitzumbo-Guzmán, R.; Bañuelos-Díaz, J.A.; Zaragoza-Contreras, E.A. Ultrasonic Dispersion and Activation of TiO2 Nanoparticles and Its Effect on Bacterial Inhibition in EVA Films. Mater. Chem. Phys. 2019, 235, 121760. [Google Scholar] [CrossRef]
- Nik, T.H.; Shahroudi, A.S.; Eraghihzadeh, Z.; Aghajani, F. Comparison of Residual Monomer Loss from Cold-Cure Orthodontic Acrylic Resins Processed by Different Polymerization Techniques. J. Orthod. 2014, 41, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Fouda, S.M.; Al-Harbi, F.A.; Näpänkangas, R.; Raustia, A. PMMA Denture Base Material Enhancement: A Review of Fiber, Filler, and Nanofiller Addition. Int. J. Nanomed. 2017, 12, 3801–3812. [Google Scholar] [CrossRef] [Green Version]
- Kanie, T.; Fujii, K.; Arikawa, H.; Inoue, K. Flexural Properties and Impact Strength of Denture Base Polymer Reinforced with Woven Glass Fibers. Dent. Mater. 2000, 16, 150–158. [Google Scholar] [CrossRef]
- Morgan, T.D.; Wilson, M. The Effects of Surface Roughness and Type of Denture Acrylic on Biofilm Formation by Streptococcus Oralis in a Constant Depth Film Fermentor. J. Appl. Microbiol. 2001, 91, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Shirkavand, S.; Moslehifard, E. Effect of TiO2 Nanoparticles on Tensile Strength of Dental Acrylic Resins. J. Dent. Res. Dent. Clin. Dent. Prospects. 2014, 8, 197–203. [Google Scholar] [CrossRef]
- Rangrazi, A.; Bagheri, H.; Ghazvini, K.; Boruziniat, A.; Darroudi, M. Synthesis and Antibacterial Activity of Colloidal Selenium Nanoparticles in Chitosan Solution: A New Antibacterial Agent. Mater. Res. Express 2020, 6, 1250h3. [Google Scholar] [CrossRef]
- Hood, M.A.; Mari, M.; Muñoz-Espí, R. Synthetic Strategies in the Preparation of Polymer/Inorganic Hybrid Nanoparticles. Materials 2014, 7, 4057–4087. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Fan, H.; Lane, J.M.D.; Qin, Y. Bottom-up Approaches for Precisely Nanostructuring Hybrid Organic/Inorganic Multi-Component Composites for Organic Photovoltaics. MRS Adv. 2020, 5, 2055–2065. [Google Scholar] [CrossRef]
- Wang, X.; Wang, P.; Jiang, Y.; Su, Q.; Zheng, J. Facile Surface Modification of Silica Nanoparticles with a Combination of Noncovalent and Covalent Methods for Composites Application. Compos. Sci. Technol. 2014, 104, 1–8. [Google Scholar] [CrossRef]
- Karci, M.; Demir, N.; Yazman, S. Evaluation of Flexural Strength of Different Denture Base Materials Reinforced with Different Nanoparticles. J. Prosthodont. 2019, 28, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Sodagar, A.; Bahador, A.; Khalil, S.; Shahroudi, A.S.; Kassaee, M.Z. The Effect of TiO2 and SiO2 Nanoparticles on Flexural Strength of Poly (Methyl Methacrylate) Acrylic Resins. J. Prosthodont. Res. 2013, 57, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Forster, A.M.; Johnson, P.M.; Eidelman, N.; Quinn, G.; Schumacher, G.; Zhang, X.; Wu, W. Improving Performance of Dental Resins by Adding Titanium Dioxide Nanoparticles. Dent. Mater. 2011, 27, 972–982. [Google Scholar] [CrossRef]
- Chatterjee, A. Effect of NanoTiO2 Addition on Poly (Methyl Methacrylate): An Exciting Nanocomposite. J. Appl. Polym. Sci. 2010, 116, 3396–3407. [Google Scholar] [CrossRef]
- Shahabi, M.; Fazel, S.M.; Rangrazi, A. Incorporation of Chitosan Nanoparticles into a Cold-Cure Orthodontic Acrylic Resin: Effects on Mechanical Properties. Biomimetics 2021, 6, 7. [Google Scholar] [CrossRef]
- Stojanovic, D.; Orlovic, A.; Markovic, S.; Radmilovic, V.; Uskokovic, P.S.; Aleksic, R. Nanosilica/PMMA Composites Obtained by the Modification of Silica Nanoparticles in a Supercritical Carbon Dioxide-Ethanol Mixture. J. Mater. Sci. 2009, 44, 6223–6232. [Google Scholar] [CrossRef]
- Soliman, T.S.; Vshivkov, S.A. Effect of Fe Nanoparticles on the Structure and Optical Properties of Polyvinyl Alcohol Nanocomposite Films. J. Non Cryst. Solids 2019, 519, 119452. [Google Scholar] [CrossRef]
- Chrysafi, I.; Kontonasaki, E.; Anastasiou, A.D.; Patsiaoura, D.; Papadopoulou, L.; Vourlias, G.; Vouvoudi, E.; Bikiaris, D. Mechanical and Thermal Properties of PMMA Resin Composites for Interim Fixed Prostheses Reinforced with Calcium β-Pyrophosphate. J. Mech. Behav. Biomed. Mater. 2020, 112, 104094. [Google Scholar] [CrossRef]
- Rudolf, R.; Popović, D.; Tomić, S.; Bobovnik, R.; Lazić, V.; Majerič, P.; Anžel, I.; Čolić, M. Microstructure Characterisation and Identification of the Mechanical and Functional Properties of a New PMMA-ZnO Composite. Materials 2020, 13, 2717. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Mbese, J.Z. Synthesis and Characterization of Metal Sulfides Nanoparticles/Poly(Methyl Methacrylate) Nanocomposites. Int. J. Polym. Sci. 2014, 2014, 752394. [Google Scholar] [CrossRef] [Green Version]













| Sample | Position (2theta) | Planes | Non-Ethanol-Assisted | Ethanol-Assisted | ||
|---|---|---|---|---|---|---|
| FWHM Left [°2Th] | Size (nm) | FWHM Left [°2Th] | Size (nm) | |||
| PMMA | 72.49 | 0.288 | 34.2 | 0.288 | 34.2 | |
| PMMA-MgO group | 42.765 | (002) | 0.315 | 27.1 | 0.315 | 27.1 |
| 62.135 | (022) | 0.236 | 39.3 | 0.236 | 39.3 | |
| 72.59 | (113) | 0.394 | 25.0 | 0.709 | 14.5 | |
| Average | 0.315 | 30.46 | 0.420 | 26.96 | ||
| PMMA- Ag group | 38.01 | (111) | 0.315 | 26.7 | 0.315 | 26.7 |
| 44.139 | (002) | 0.551 | 15.6 | 0.63 | 13.6 | |
| 64.355 | (022) | 0.315 | 29.8 | 0.472 | 19.9 | |
| 77.26 | (113) | 0.394 | 25.8 | 0.63 | 16.1 | |
| Average | 0.3937 | 24.47 | 0.5117 | 19.07 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arf, A.N.; Kareem, F.A.; Gul, S.S. Dispersion and Homogeneity of MgO and Ag Nanoparticles Mixed with Polymethylmethacrylate. Polymers 2023, 15, 1479. https://doi.org/10.3390/polym15061479
Arf AN, Kareem FA, Gul SS. Dispersion and Homogeneity of MgO and Ag Nanoparticles Mixed with Polymethylmethacrylate. Polymers. 2023; 15(6):1479. https://doi.org/10.3390/polym15061479
Chicago/Turabian StyleArf, Awder Nuree, Fadil Abdullah Kareem, and Sarhang Sarwat Gul. 2023. "Dispersion and Homogeneity of MgO and Ag Nanoparticles Mixed with Polymethylmethacrylate" Polymers 15, no. 6: 1479. https://doi.org/10.3390/polym15061479
APA StyleArf, A. N., Kareem, F. A., & Gul, S. S. (2023). Dispersion and Homogeneity of MgO and Ag Nanoparticles Mixed with Polymethylmethacrylate. Polymers, 15(6), 1479. https://doi.org/10.3390/polym15061479