Synthesis and Design of Hybrid Metalloporphyrin Polymers Based on Palladium (II) and Copper (II) Cations and Axial Complexes of Pyridyl-Substituted Sn(IV)Porphyrins with Octopamine
Abstract
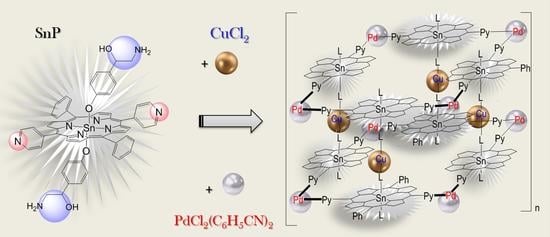
:1. Introduction
2. Experimental
2.1. Equipment
2.2. Computational Details
2.3. Synthesis


2.4. DOSY NMR Spectroscopy
2.5. Photoresistance and Thermal Stability Study Technique
2.6. Determination of the Porosity of Hybrid Sn(IV)-Porphyrin Polymers
3. Results and Discussion
3.1. Structures of the Sn(IV)-Dipyridylporphyrin Based Systems
3.2. Powder X-ray Diffraction Studies and Thermogravimetric Analysis
3.3. Determination of the Porosity of the Hybrid Sn(IV)-Porphyrin Polymers
3.4. Photo- and Thermal Stability of Obtained Poly-Sn(IV)Porphyrin Systems in Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lv, N.; Li, Q.; Zhu, H.; Mu, S.; Luo, X.; Ren, X.; Liu, X.; Li, S.; Cheng, C.; Ma, T. Electrocatalytic Porphyrin/Phthalocyanine-Based Organic Frameworks: Building Blocks, Coordination Microenvironments, Structure-Performance Relationships. Adv. Sci. 2023, 2206239. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.-Y.; Joseph, E.; Zhou, H.-C. Catalytic Porphyrin Framework Compounds. Trends Chem. 2020, 2, 555–568. [Google Scholar] [CrossRef]
- Gu, J.; Peng, Y.; Zhou, T.; Ma, J.; Pang, H.; Yamauchi, Y. Porphyrin-based framework materials for energy conversion. Nano Res. Energy 2022, 1, 9120009. [Google Scholar] [CrossRef]
- Ma, L.; Arman, H.; Xie, Y.; Zhou, W.; Chen, B. Solvent-Dependent Self-Assembly of Hydrogen-Bonded Organic Porphyrinic Frameworks. Cryst.Growth Des. 2022, 22, 3808–3814. [Google Scholar] [CrossRef]
- Benseghir, Y.; Solé-Daura, A.; Cairnie, D.R.; Robinson, A.L.; Duguet, M.; Mialane, P.; Gairola, P.; Gomez-Mingot, M.; Fontecave, M.; Iovan, D.; et al. Unveiling the mechanism of the photocatalytic reduction of CO2 to formate promoted by porphyrinic Zr-based metal–organic frameworks. J. Mater. Chem.A 2022, 10, 18103–18115. [Google Scholar] [CrossRef]
- Li, X.; Yao, Q.; Li, Z.; Li, H.; Zhu, Q.-L.; Lu, Z.H. Porphyrin framework-derived N-doped porous carbon-confined Ru for NH3BH3methanolysis: The more pyridinic-N, the better. J. Mater. Chem. A 2022, 10, 326–336. [Google Scholar]
- Xiao, Y.-H.; Zhang, Y.-X.; Zhai, R.; Gu, Z.-G.; Zhang, J. Helical copper-porphyrinic framework nanoarrays for highly efficient CO2 electroreduction. Sci. China Mater. 2022, 65, 1269–1275. [Google Scholar] [CrossRef]
- Das, R.; Manna, S.S.; Pathak, B.; Nagaraja, C.M. Strategic Design of Mg-Centered Porphyrin Metal-Organic Framework for Efficient Visible Light-Promoted Fixation of CO2 under Ambient Conditions: Combined Experimental and Theoretical Investigation. ACS Appl. Mater. Interfaces 2022, 14, 33285–33296. [Google Scholar] [CrossRef]
- Xia, Z.; Yu, R.; Yang, H.; Luo, B.; Huang, Y.; Li, D.; Shi, J.; Xu, D. Novel 2D Zn-porphyrin metal organic frameworks revived CdS for photocatalysis of hydrogen production. Int. J. Hydrogen Energy 2022, 47, 13340–13350. [Google Scholar] [CrossRef]
- Ma, C.; Wolterbeek, H.T.; Denkova, A.G.; Crespo, P.S. Porphyrinic metal–organic frameworks as molybdenum adsorbents for the 99Mo/99mTc generator. Inorg. Chem. Front. 2023. [Google Scholar] [CrossRef]
- Lv, F.; Sun, M.; Hu, Y.; Xu, J.; Huang, W.; Han, N.; Huang, B.; Li, Y. Near-unity electrochemical conversion of nitrate to ammonia on crystalline nickel porphyrin-based covalent organic frameworks. Energy Environ. Sci. 2023, 16, 201–209. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.-P.; Lei, H.; Guo, K.; Xu, G.; Xie, L.; Li, X.; Zhang, W.; Apfel, U.-P.; Cao, R. Tuning Electronic Structures of Covalent Co Porphyrin Polymers for Electrocatalytic CO2 Reduction in Aqueous Solutions. CCS Chem. 2022, 4, 2959–2967. [Google Scholar] [CrossRef]
- Jin, F.; Lin, E.; Wang, T.; Yan, D.; Yang, Y.; Chen, Y.; Cheng, P.; Zhang, Z. Rationally fabricating 3D porphyrinic covalent organic frameworks with scu topology as highly efficient photocatalysts. Chem 2022, 8, 3064–3080. [Google Scholar] [CrossRef]
- Chi, S.-Y.; Chen, Q.; Zhao, S.S.; Si, D.-H.; Wu, Q.-J.; Huang, Y.-B.; Cao, R. Three-dimensional porphyrinic covalent organic frameworks for highly efficient electroreduction of carbon dioxide. J. Mater. Chem. A 2022, 10, 4653–4659. [Google Scholar] [CrossRef]
- Paille, G.; Gomez-Mingot, M.; Roch-Marchal, C.; Lassalle-Kaiser, B.; Mialane, P.; Fontecave, M.; Mellot-Draznieks, C.; Dolbecq, A. A Fully Noble Metal-Free Photosystem Based on Cobalt-Polyoxometalates Immobilized in a Porphyrinic Metal–Organic Framework for Water Oxidation. J. Am. Chem. Soc. 2018, 140, 3613–3618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucheryavy, P.; Lahanas, N.; Lockard, J.V. Spectroscopic Evidence of Pore Geometry Effect on Axial Coordination of Guest Molecules in Metalloporphyrin-Based Metal Organic Frameworks. Inorg. Chem. 2018, 57, 3339–3347. [Google Scholar] [CrossRef]
- Stassen, I.; Burtch, N.; Talin, A.; Falcaro, P.; Allendorf, M.; Ameloot, R. An updated roadmap for the integration of metal–organic frameworks with electronic devices and chemical sensors. Chem. Soc. Rev. 2017, 46, 3185–3241. [Google Scholar] [CrossRef]
- Huh, S.; Kim, S.-J.; Kim, Y. Porphyrinic metal–organic frameworks from custom-designed porphyrins. CrystEngComm 2016, 18, 345–368. [Google Scholar] [CrossRef]
- Day, N.U.; Wamser, C.C.; Walter, M.G. Porphyrin polymers and organic frameworks. Polym. Int. 2015, 64, 833–857. [Google Scholar] [CrossRef]
- Ermakova, E.V.; Enakieva, Y.Y.; Meshkov, I.N.; Baranchikov, A.E.; Zvyagina, A.I.; Gorbunova, Y.G.; Tsivadze, A.Y.; Kalinina, M.A.; Arslanov, V.V. Bilayer Porphyrin-Graphene Templates for Self-Assembly of Metal-Organic Frameworks on the Surface. Macroheterocycles 2017, 10, 496–504. [Google Scholar] [CrossRef] [Green Version]
- Gilday, L.C.; White, N.; Beer, P.D. Halogen- and hydrogen-bonding triazole-functionalised porphyrin-based receptors for anion recognition. Dalton Trans. 2013, 42, 15766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamardashvili, G.M.; Mamardashvili, N.Z. Self-organization of zinc(II) and tin(IV) porphyrinates into supramolecular trimers. Russ. J. Gen. Chem. 2013, 83, 1424–1428. [Google Scholar] [CrossRef]
- Khodov, I.; Alper, G.; Mamardashvili, G.; Mamardashvili, N. Hybrid multi-porphyrin supramolecular assemblies: Synthesis and structure elucidation by 2D DOSY NMR studies. J. Mol. Struct. 2015, 1099, 174–180. [Google Scholar] [CrossRef]
- Mamardashvili, G.M.; Lazovskiy, D.A.; Khodov, I.A.; Efimov, A.E.; Mamardashvili, N.Z. New Polyporphyrin Arrays with Controlled Fluorescence Obtained by Diaxial Sn(IV)-Porphyrin Phenolates Chelation with Cu2+ Cation. Polymers 2021, 13, 829. [Google Scholar] [CrossRef] [PubMed]
- Likhonina, A.E.; Lebedev, I.S.; Mamardashvili, G.M.; Mamardashvili, N.Z. pH Indicator and Rotary Fluorescent Properties of the Sn(IV)-octaetylporphyrin-(BODIPY)2 Triad. Inorg. Chim. Acta 2022, 542, 121150. [Google Scholar] [CrossRef]
- Likhonna, A.E.; Mamardashvili, G.M.; Mamardashvili, N.Z. Photoactive porphyrin-fluorescein arrays to control the acidity of medium. J. Photochem. Photobiol. A Chem. 2022, 424, 113650. [Google Scholar] [CrossRef]
- Czaja, A.U.; Trukhan, N.; Müller, U. Industrial applications of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1284–1293. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Li, Y.; Ban, Y.; Jin, H.; Jiao, W.; Liu, X.; Yang, W. Metal-organic framework nanosheets as building blocks for molecular sieving membranes. Science 2014, 346, 1356–1359. [Google Scholar] [CrossRef]
- Falcaro, P.; Ricco, R.; Doherty, C.M.; Liang, K.; Hill, A.J.; Styles, M.J. MOF positioning technology and device fabrication. Chem. Soc. Rev. 2014, 43, 5513–5560. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-N.; Wang, S.; Zhou, Y.; Bai, X.-J.; Song, G.-S.; Zhao, X.-Y.; Wang, T.-Q.; Qi, X.; Zhang, X.-M.; Fu, Y. Fabrication of Metal–Organic Framework and Infinite Coordination Polymer Nanosheets by the Spray Technique. Langmuir 2017, 33, 1060–1065. [Google Scholar] [CrossRef]
- Avezov, K.G.; Umarov, B.B.; Tursunov, M.A.; Parpiev, N.A.; Minin, V.V. Copper(II) complexes based on 2-thenoyltrifluoroacetone aroylhydrazones: Synthesis, spectroscopt and X-ray diffraction analysis. Russ. J. Coord. Chem. 2016, 42, 470–475. [Google Scholar] [CrossRef]
- Timmerman, P.; Weidmann, J.-L.; Jolliffe, K.A.; Prins, L.J.; Reinhoudt, D.N.; Shinkai, S.; Frish, L.; Cohen, Y. NMR diffusion spectroscopy for the characterization of multicomponent hydrogen-bonded assemblies in solution. J. Chem. Soc. Perkin Trans. 2 2000, 2, 2077–2089. [Google Scholar] [CrossRef]
- Ding, Q.; Xu, Z.; Zhou, L.; Rao, C.; Li, W.; Muddassir, M.; Sakiyama, H.; Li, B.; Ouyang, Q.; Liu, J. A multimodal Metal-Organic framework based on unsaturated metal site for enhancing antitumor cytotoxicity through Chemo-Photodynamic therapy. J. Colloid. Interf. Sci. 2022, 621, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Ma, D. A 2D porous zinc-organic framework platform for loading of 5-fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Ma, D. A microporous 2D cobalt-based MOF with pyridyl sites and open metal sites for selective adsorption of CO2. Microporous Mesoporous Mater. 2022, 341, 112098. [Google Scholar] [CrossRef]
- Dong, X.; Shi, Z.; Li, D.; Li, Y.; An, N.; Shang, Y.; Si, C. The regulation research of topology and magnetic exchange models of CPs through Co (II) concentration adjustment. J. Solid State Chem. 2023, 318, 123713. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Rassolov, V.A.; Ratner, M.A.; Pople, J.A.; Redfern, P.C.; Curtiss, L.A. 6-31G* basis set for third-row atoms. J. Comput. Chem. 2001, 22, 976–984. [Google Scholar] [CrossRef]
- Cammi, R.; Tomasi, J. Remarks on the use of the apparent surface charges (ASC) methods in solvation problems: Iterative versus matrix-inversion procedures and the renormalization of the apparent charges. J. Comput. Chem. 1995, 16, 1449–1458. [Google Scholar] [CrossRef]
- Chemcraft—Graphical Software for Visualization of Quantum Chemistry Computations. Available online: https://www.chemcraftprog.com (accessed on 22 December 2022).
- Aguilar, J.A.; Nilsson, M.; Bodenhausen, G.; Morris, G.A. Spin echo NMR spectra without J modulation. Chem. Commun. 2012, 48, 811–813. [Google Scholar] [CrossRef] [Green Version]
- Barton, R.H.; Waterman, D.; Bonner, F.W.; Holmes, E.; Clarke, R.; The PROCARDIS Consortium; Nicholsona, J.K.; Lindon, J.C. The influence of EDTA and citrate anticoagulant addition to human plasma on information recovery from NMR-based metabolic profiling studies. Mol. BioSyst. 2010, 6, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Hu¨rlimann, M.D.; Griffin, D.D. Spin Dynamics of Carr–Purcell–Meiboom–Gill-like Sequences in Grossly Inhomogeneous B0 and B1 Fields and Application to NMR Well Logging. J. Magn. Reson. 2000, 143, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Jarenwattananon, N.N.; Bouchard, L.-S. Breakdown of Carr-Purcell Meiboom-Gill spin echoes in inhomogeneous fields. J. Chem. Phys. 2018, 149, 084304. [Google Scholar] [CrossRef]
- Khodov, I.A. Accuracy of determination of self-diffusion coefficients in studies of porphyrin-based complexes by 2D DOSY. Macroheterocycles 2017, 10, 313–316. [Google Scholar] [CrossRef] [Green Version]
- Ksenofontov, A.A.; Lukanov, M.M.; Bichan, N.G.; Khodov, I.A.; Kudryakova, N.O.; Ksenofontova, K.V.; Antina, E.V. Non-covalent supramolecular systems with photoinduced electron transfer based on zinc bis(dipyrromethenate)s and C60. Dyes Pigment. 2021, 185, 108918. [Google Scholar] [CrossRef]
- Ksenofontov, A.A.; Stupikova, S.A.; Bocharov, P.S.; Lukanov, M.M.; Ksenofontova, K.V.; Khodov, I.A.; Antina, E.V. Novel fluorescent sensors based on zinc(II) bis(dipyrromethenate)s for furosemide detection in organic media. J. Photochem. Photobiol. A Chem. 2019, 382, 11189. [Google Scholar] [CrossRef]
- Oliva, A.I.; Gómez, K.; Gonzáleza, G.; Ballester, P. Diffusion-ordered spectroscopy (1 H-DOSY) of Zn-porphyrin assemblies induced by coordination with DABCO. New J. Chem. 2008, 32, 2159–2163. [Google Scholar] [CrossRef]
- Holz, M.; Mao, X.; Seiferling, D.; Sacco, A. Experimental study of dynamic isotope effects in molecular liquids: Detection of translationrotation coupling. J. Chem. Phys. 1996, 104, 669. [Google Scholar] [CrossRef]
- Waldeck, A.R.; Kuche, P.W.; Lennon, A.J.; Chapman, B.E. NMR diffusion measurements to characterise membrane transport and solute binding. Prog. Nucl. Magn. Reson. Spectrosc. 1997, 30, 39–68. [Google Scholar] [CrossRef]
- Ikeda, A.; Ayabe, M.; Shinkai, S.; Sakamoto, S.; Yamaguchi, K. A Self-Assembled Porphyrin-Based Dimeric Capsule Constructed by a Pd(II)-Pyridine Interaction Which Shows Efficient Guest Inclusion. Org. Lett. 2000, 2, 3707–3710. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva, O.A.; Mamardashvili, N.Z. Porphyrin dimer as efficient optical thermometer: Experimental and computational evaluation of the barrier to torsional rotation. J. Lumin. 2021, 235, 117986. [Google Scholar] [CrossRef]












| Name | Number of Monomer Units | Dexp (×10−9) (m2/s) | Molecular Weight (g/mol) |
|---|---|---|---|
| Sn(OH)2P 1 | Monomer | 0.83 | 767.42 |
| (Sn(OH)2P)2PdCl2 5 | Dimer | 0.73 | 1712.26 |
| (Sn(OH)2P)4(PdCl2)4 2 | Tetramer | 0.58 | 3779.36 |
| Name | Number of Monomer Units | Dexp (×10−10) (m2/s) | Molecular Weight (g/mol) |
|---|---|---|---|
| DMSO | Reference | 4.13 | 84.17 |
| (Sn(OH)2P)4(PdCl2)4 2 | Mono- | 0.83 | 3779.36 |
| Di- | 0.75 | 7558.72 | |
| Three- | 0.64 | 11,338.08 | |
| Tetra- | 0.56 | 34,014.24 |
| Name | Number of Monomer Units | Dexp (×10−10) (m2/s) | Molecular Weight (g/mol) |
|---|---|---|---|
| DMSO | Reference | 5.21 | 84.17 |
| (Sn(L)2P)4(PdCl2)4 3 | Mono- | 0.86 | 5152.345 |
| Name | Number of Monomer Units (n) | Dexp (×10−10) (m2/s) | Molecular Weight (g/mol) |
|---|---|---|---|
| CDCl3 | Reference | 19.4 | 119.38 |
| ((Sn(L-Cu)2P)4(PdCl2)4)n 4 | Di- | 4.32 | 9704.69 |
| Hexa- | 2.71 | 29,114.07 | |
| Octa- | 2.61 | 38,818.76 | |
| Deca- | 1.59 | 48,523.45 |
| Name | r(Sn-O), Å | Est(Sn-O), kJ/mol | qst,e | r(Sn-Np),Å | Est(Sn-Np), kJ/mol | qst,e | <L-O-O-L, ° | <Sn-O-L, ° |
|---|---|---|---|---|---|---|---|---|
| Sn(OH)2P 1 | 1.998 | 451.9 | 0.481 | 2.101 | 397.5 | 0.521 | 78.3 | 116.3 |
| Sn(L)2P 6 | 2.033 | 230.4 | 0.608 | 2.098 | 195.2 | 0.455 | 129.0 | 132.1 |
| Name | r(Sn-O),Å | Est(Sn-O)kJ/mol | qst,e | r(Sn-Np),Å | Est(Sn-Np), kJ/mol | qst,e | <Sn-O-L,° | r(Sn-Sn),Å |
|---|---|---|---|---|---|---|---|---|
| (Sn(L)2P)2Cu 7 | 2.038 | 207.1 | 0.446 | 2.091 | 242.9 | 0.678 | 122.5 | 18.124 |
| Name Parameters | (Sn(OH)2P)4(PdCl2)4 2 | (Sn(L)2P)4(PdCl2)4 3 |
|---|---|---|
| r(Sn-O), Ǻ | 2.014 | 2.011 |
| r(Sn-N), Ǻ | 2.112 | 2.108 |
| <Sn-O-L, ° | 114.1 | 114.6 |
| d(Sn…Sn) X-axis * | 18.98 | 19.23 |
| d(Sn…Sn) Y-axis ** | 18.84 | 19.01 |
| Name | Adsorption | Desorption | ||||
|---|---|---|---|---|---|---|
| S,m2/g | V, cm3/г | r, nm | S,m2/g | V, cm3/g | r, nm | |
| BJH | ||||||
| Sn(OH)2P 1 | 154.686 | 0.27 | 1.68 | 192.71 | 0.31 | 1.53 |
| Sn(L)2P 6 | 257.868 | 0.38 | 1.88 | 334.319 | 0.48 | 2.15 |
| (Sn(L)2P)2Cu 7 | 214.574 | 0.35 | 2.11 | 238.272 | 0.39 | 1.91 |
| (Sn(OH)2P)4(PdCl2)4, 2 | 3420.48 | 5.41 | 1.69 | 3699.60 | 5.89 | 1.70 |
| (Sn(L)2P)4(PdCl2)4, 3 | 2340.82 | 3.99 | 1.89 | 2865.23 | 4.59 | 1.89 |
| ((Sn(L-Cu)2P)4(PdCl2)4)n, 4 | 2694.52 | 4.28 | 1.90 | 3190.07 | 4.85 | 1.90 |
| BET | ||||||
| Sn(OH)2P 1 | 158.278 | 0.26 | 1.68 | 197.177 | 0.30 | 1.53 |
| Sn(L)2P 6 | 263.959 | 0.37 | 1.88 | 342.071 | 0.47 | 2.15 |
| (Sn(L)2P)2Cu 7 | 219.609 | 0.35 | 2.11 | 243.848 | 0.38 | 1.91 |
| (Sn(OH)2P)4(PdCl2)4, 2 | 3499.82 | 5.30 | 1.69 | 3786.12 | 5.77 | 1.70 |
| (Sn(L)2P)4(PdCl2)4, 3 | 2462.23 | 3.89 | 1.89 | 2932.47 | 4.49 | 1.70 |
| ((Sn(L-Cu)2P)4(PdCl2)4)n, 4 | 2757.43 | 4.19 | 1.90 | 3183.41 | 4.75 | 1.90 |
| Name | η, % | k, min−1 | τ1/2, min |
|---|---|---|---|
| Sn(OH)2P 1 | 15 | 0.0021 | 237 |
| (Sn(OH)2P)4(PdCl2)4 2 | 11 | 0.0015 | 462 |
| (Sn(L)2P)4(PdCl2)4 3 | 4 | 0.0004 | 1733 |
| ((Sn(L-Cu)2P)4(PdCl2)4)n 4 | 3 | 0.0005 | 1386 |
| Sn(L)2P 6 | 7 | 0.0009 | 893 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Likhonina, A.E.; Mamardashvili, G.M.; Khodov, I.A.; Mamardashvili, N.Z. Synthesis and Design of Hybrid Metalloporphyrin Polymers Based on Palladium (II) and Copper (II) Cations and Axial Complexes of Pyridyl-Substituted Sn(IV)Porphyrins with Octopamine. Polymers 2023, 15, 1055. https://doi.org/10.3390/polym15041055
Likhonina AE, Mamardashvili GM, Khodov IA, Mamardashvili NZ. Synthesis and Design of Hybrid Metalloporphyrin Polymers Based on Palladium (II) and Copper (II) Cations and Axial Complexes of Pyridyl-Substituted Sn(IV)Porphyrins with Octopamine. Polymers. 2023; 15(4):1055. https://doi.org/10.3390/polym15041055
Chicago/Turabian StyleLikhonina, Anastasia E., Galina M. Mamardashvili, Ilya A. Khodov, and Nugzar Z. Mamardashvili. 2023. "Synthesis and Design of Hybrid Metalloporphyrin Polymers Based on Palladium (II) and Copper (II) Cations and Axial Complexes of Pyridyl-Substituted Sn(IV)Porphyrins with Octopamine" Polymers 15, no. 4: 1055. https://doi.org/10.3390/polym15041055
APA StyleLikhonina, A. E., Mamardashvili, G. M., Khodov, I. A., & Mamardashvili, N. Z. (2023). Synthesis and Design of Hybrid Metalloporphyrin Polymers Based on Palladium (II) and Copper (II) Cations and Axial Complexes of Pyridyl-Substituted Sn(IV)Porphyrins with Octopamine. Polymers, 15(4), 1055. https://doi.org/10.3390/polym15041055