Physicochemical Properties of UV-Irradiated, Biaxially Oriented PLA Tubular Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
2.1. PLA Tube Fabrication and Sample Preparation
2.2. Fabrication of Biaxially Expanded Tubular Scaffolds
2.3. UV Irradiation
2.4. Characterization Techniques
3. Results and Discussion
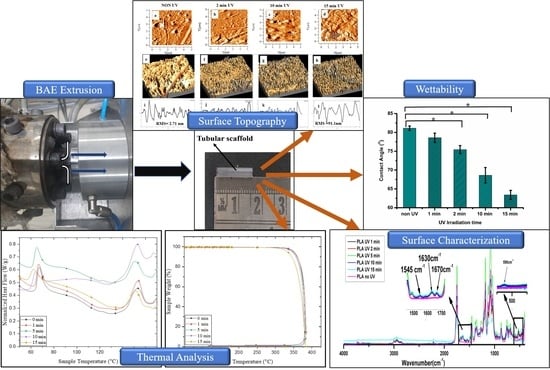
3.1. Physicochemical Properties of PLA-UV
3.2. XPS
3.3. Thermal Stability
3.4. Mechanical Characterization—Radial Compressive Testing
3.5. Surface Wettability
3.6. Morphological Characterization
3.6.1. SEM Analysis
3.6.2. AFM Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, L.-T.; Auras, R.; Rubino, M. Processing Technologies for Poly (Lactic Acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Krishnan, S.; Pandey, P.; Mohanty, S.; Nayak, S.K. Toughening of Polylactic Acid: An Overview of Research Progress. Polym. Plast. Technol. Eng. 2016, 55, 1623–1652. [Google Scholar] [CrossRef]
- Srivastava, A.; Bhatnagar, N. Production and Characterisation of New Bioresorbable Radiopaque Mg-Zn-Y Alloy to Improve X-Ray Visibility of Polymeric Scaffolds. J. Magnes. Alloy. 2022, 10, 1694–1703. [Google Scholar] [CrossRef]
- Ahuja, R.; Kumari, N.; Srivastava, A.; Bhati, P.; Vashisth, P.; Yadav, P.K.; Jacob, T.; Narang, R.; Bhatnagar, N. Biocompatibility Analysis of PLA Based Candidate Materials for Cardiovascular Stents in a Rat Subcutaneous Implant Model. Acta Histochem. 2020, 122, 151615. [Google Scholar] [CrossRef] [PubMed]
- Pukhova, I.V.; Savkin, K.P.; Laput, O.A.; Lytkina, D.N.; Botvin, V.V.; Medovnik, A.V.; Kurzina, I.A. Effects of Ion- and Electron-Beam Treatment on Surface Physicochemical Properties of Polylactic Acid. Appl. Surf. Sci. 2017, 422, 856–862. [Google Scholar] [CrossRef]
- Rudolph, A.; Teske, M.; Illner, S.; Kiefel, V.; Sternberg, K.; Grabow, N.; Wree, A.; Hovakimyan, M. Surface Modification of Biodegradable Polymers towards Better Biocompatibility and Lower Thrombogenicity. PLoS ONE 2015, 10, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Riveiro, A.; Maçon, A.L.B.; del Val, J.; Comesaña, R.; Pou, J. Laser Surface Texturing of Polymers for Biomedical Applications. Front. Phys. 2018, 5, 16. [Google Scholar] [CrossRef]
- Koo, G.; Jang, J. Surface Modification of Poly (Lactic Acid) by UV/Ozone Irradiation. Fibers Polym. 2008, 9, 674–678. [Google Scholar] [CrossRef]
- Kowalonek, J.; Vuković-Kwiatkowska, I.; Moszyński, D.; Kaczmarek, H. Surface Properties of Poly(Lactic Acid)/Polyacrylate Semi-Interpenetrating Networks—Effect of UVC Radiation. Polym. Degrad. Stab. 2016, 131, 71–81. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E. Effect of UV Irradiation on Thermal Properties of Nanocomposites Based on Polylactide. J. Therm. Anal. Calorim. 2015, 119, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Ho, K.-L.G.; Pometto Iii, A.L. Effects of Electron-Beam Irradiation and Ultraviolet Light (365 Nm) on Polylactic Acid Plastic Films. J. Environ. Polym. Degrad. 1999, 7, 93–100. [Google Scholar] [CrossRef]
- Podzorova, M.V.; Tertyshnaya, Y.V.; Pantyukhov, P.V.; Popov, A.A.; Nikolaeva, S.G. Influence of Ultraviolet on Polylactide Degradation. In Proceedings of the AIP Conference Proceedings, Tomsk, Russia, 1 December 2017; American Institute of Physics Inc.: College Park, MD, USA, 2017; Volume 1909. [Google Scholar]
- Copinet, A.; Bertrand, C.; Govindin, S.; Coma, V.; Couturier, Y. Effects of Ultraviolet Light (315 Nm), Temperature and Relative Humidity on the Degradation of Polylactic Acid Plastic Films. Chemosphere 2004, 55, 763–773. [Google Scholar] [CrossRef]
- Bhati, P.; Ahuja, R.; Srivastava, A.; Pankaj; Singh, S.; Vashisth, P.; Bhatnagar, N. Physicochemical Characterization and Mechanical Performance Analysis of Biaxially Oriented PLA/PCL Tubular Scaffolds for Intended Stent Application. SN Appl. Sci. 2020, 2, 2089. [Google Scholar] [CrossRef]
- Bhati, P.; Kumar, A.; Ahuja, R.; Bhatnagar, N. Evaluating the Effect of Manufacturing Method on the Radial Compressive Force of the Bioresorbable Tubes. Mater. Lett. 2019, 235, 23–26. [Google Scholar] [CrossRef]
- Bai, H.; Xiu, H.; Gao, J.; Deng, H.; Zhang, Q.; Yang, M.; Fu, Q. Tailoring Impact Toughness of Poly(L-Lactide)/Poly(??-Caprolactone) (PLLA/PCL) Blends by Controlling Crystallization of PLLA Matrix. ACS Appl. Mater. Interfaces 2012, 4, 897–905. [Google Scholar] [CrossRef]
- Bhatnagar, N.; Bhati, P. Polymer Tubes for Manufacturing Stents. Indian Patent IN201611035281A, 14 October 2016. [Google Scholar]
- Brownson, J.R.S. Laws of Light. In Solar Energy Conversion Systems; Elsevier: Amsterdam, The Netherlands, 2014; pp. 41–66. [Google Scholar]
- Xiuwen, X. Exchanging and Application between Nomogram Determining Exposure Time and Exposing Curve after Variations of Focal Distance. In Non-Destructive Testing ’92; Hallai, C., Kulcsar, P., Eds.; Elsevier: Oxford, UK, 1992; pp. 692–696. ISBN 978-0-444-89791-6. [Google Scholar]
- Mills, D.; Harnish, D.A.; Lawrence, C.; Sandoval-Powers, M.; Heimbuch, B.K. Ultraviolet Germicidal Irradiation of Influenza-Contaminated N95 Filtering Facepiece Respirators. Am. J. Infect. Control 2018, 46, e49–e55. [Google Scholar] [CrossRef] [Green Version]
- Bocchini, S.; Fukushima, K.; di Blasio, A.; Fina, A.; Frache, A.; Geobaldo, F. Polylactic Acid and Polylactic Acid-Based Nanocomposite Photooxidation. Biomacromolecules 2010, 11, 2919–2926. [Google Scholar] [CrossRef]
- Gardette, M.; Thérias, S.; Gardette, J.L.; Murariu, M.; Dubois, P. Photooxidation of Polylactide/Calcium Sulphate Composites. Polym. Degrad. Stab. 2011, 96, 616–623. [Google Scholar] [CrossRef]
- Rasselet, D.; Ruellan, A.; Guinault, A.; Miquelard-garnier, G.; Sollogoub, C. Oxidative Degradation of Polylactide (PLA) and Its Effects on Physical and Mechanical Properties. Eur. Polym. J. 2014, 50, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Kister, G.; Cassanas, G.; Vert, M. Effects of Morphology, Conformation and Configuration on the IR and Raman Spectra of Various Poly(Lactic Acid)s. Polymer 1998, 39, 267–273. [Google Scholar] [CrossRef]
- Moura, I.; Botelho, G.; Machado, A.V. Characterization of EVA/PLA Blends When Exposed to Different Environments. J. Polym. Environ. 2014, 22, 148–157. [Google Scholar] [CrossRef]
- Smita Mohanty, M.C. Effect of Different Solvents in Solvent Casting of Porous Caffolds—In Biomedical and Tissue Engineering Applications. J. Tissue Sci. Eng. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Kiss, E.; Bertóti, I.; Vargha-Butler, E.I. XPS and Wettability Characterization of Modified Poly(Lactic Acid) and Poly(Lactic/Glycolic Acid) Films. J. Colloid. Interface Sci. 2002, 245, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Paragkumar, N.T.; Edith, D.; Six, J.-L. Surface Characteristics of PLA and PLGA Films. Appl. Surf. Sci. 2006, 253, 2758–2764. [Google Scholar] [CrossRef]
- Werner, W.S.M.; Briggs, D.; Grant, J. Surface Analysis by Auger and X-Ray Photoelectron Spectroscopy; IM Publications: Chichester, UK, 2003; Chapter 5; ISBN 978-1-901019-04-9. [Google Scholar]
- Lumeau, J.; Glebova, L.; Glebov, L.B. Influence of UV-Exposure on the Crystallization and Optical Properties of Photo-Thermo-Refractive Glass. J. Non. Cryst. Solids 2008, 354, 425–430. [Google Scholar] [CrossRef]
- Bhati, P.; Bhatnagar, N. Effect of Processing Parameters on Surface Hydrophilicity of Porous PLA Tubes Prepared by Gas Assisted Microcellular Extrusion Foaming Technique. Mater. Lett. 2017, 209, 602–605. [Google Scholar] [CrossRef]
- Ren, Y.; Hu, J.; Yang, M.; Weng, Y. Biodegradation Behavior of Poly (Lactic Acid) (PLA), Poly (Butylene Adipate-Co-Terephthalate) (PBAT), and Their Blends Under Digested Sludge Conditions. J. Polym. Environ. 2019, 27, 2784–2792. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, Z.; Guo, G. Biodegradation Assessment of Poly (Lactic Acid) Filled with Functionalized Titania Nanoparticles (PLA/TiO2) under Compost Conditions. Nanoscale Res. Lett. 2019, 14, 56. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek, H.; Nowicki, M.; Vuković-Kwiatkowska, I.; Nowakowska, S. Crosslinked Blends of Poly(Lactic Acid) and Polyacrylates: AFM, DSC and XRD Studies. J. Polym. Res. 2013, 20, 91. [Google Scholar] [CrossRef] [Green Version]
- Borcia, C.; Borcia, G.; Dumitrascu, N. Surface Treatment of Polymers by Plasma and UV Radiation. Rom. J. Phys. 2011, 56, 224–232. [Google Scholar]










| Time (min) | 1 | 2 | 5 | 10 | 15 |
| UV doses (J/cm2) | 1887 | 3774 | 9436 | 18,872 | 28,308 |
| Wavenumber (cm−1) | Functional Group |
|---|---|
| 1750 | (C=O) stretching |
| 1450 | (C-H) stretching |
| 1180 | C-C(O)-C stretching |
| 1080 | (C-O) stretching |
| 867 | (C-COO) stretching |
| UV-Treated PLA Sample (min) | Tg (°C) | Tm (°C) | ΔHm (W/g) | χc (%) | T10 (°C) |
|---|---|---|---|---|---|
| 0 | 67.1 | 152.7 | 15.1 | 16.9 | 360.01 |
| 1 | 66.8 | 152.3 | 18.1 | 20.3 | 359.93 |
| 5 | 65.1 | 151.9 | 16.0 | 17.9 | 357.7 |
| 10 | 64.1 | 151.5 | 14.9 | 16.7 | 355.85 |
| 15 | 62.61 | 150.2 | 14.1 | 15.8 | 342.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhati, P.; Srivastava, A.; Ahuja, R.; Chauhan, P.; Vashisth, P.; Bhatnagar, N. Physicochemical Properties of UV-Irradiated, Biaxially Oriented PLA Tubular Scaffolds. Polymers 2023, 15, 1097. https://doi.org/10.3390/polym15051097
Bhati P, Srivastava A, Ahuja R, Chauhan P, Vashisth P, Bhatnagar N. Physicochemical Properties of UV-Irradiated, Biaxially Oriented PLA Tubular Scaffolds. Polymers. 2023; 15(5):1097. https://doi.org/10.3390/polym15051097
Chicago/Turabian StyleBhati, Pooja, Alok Srivastava, Ramya Ahuja, Pankaj Chauhan, Priya Vashisth, and Naresh Bhatnagar. 2023. "Physicochemical Properties of UV-Irradiated, Biaxially Oriented PLA Tubular Scaffolds" Polymers 15, no. 5: 1097. https://doi.org/10.3390/polym15051097
APA StyleBhati, P., Srivastava, A., Ahuja, R., Chauhan, P., Vashisth, P., & Bhatnagar, N. (2023). Physicochemical Properties of UV-Irradiated, Biaxially Oriented PLA Tubular Scaffolds. Polymers, 15(5), 1097. https://doi.org/10.3390/polym15051097