Nanostructuring Biobased Epoxy Resin with PEO-PPO-PEO Block Copolymer
Abstract
:1. Introduction
2. Materials and Methods
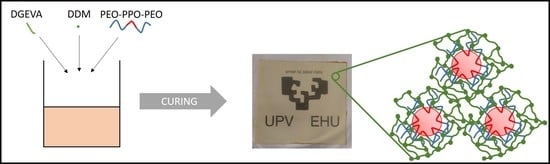
2.1. Materials and Sample Preparation
2.2. Techniques
3. Results and Discussion
3.1. Differential Scaning Calorimetry Analysis
3.2. Thermogravimetric Analysis
3.3. Fourier-Transform Infrared Spectroscopy Analysis
3.4. Atomic Force Microscopy
3.5. UV-Vis Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benyahya, S.; Aouf, C.; Caillol, S.; Boutevin, B.; Pascault, J.P.; Fulcrand, H. Functionalized green tea tannins as phenol-ic prepolymers for bio-based epoxy resins. Ind. Crops Prod. 2014, 53, 296–307. [Google Scholar] [CrossRef]
- Ma, S.; Liu, X.; Jiang, Y.; Zhang, C.; Zhu, J. Bio-based epoxy resin from itaconic acid and its thermosets cured with an-hydride and comonomers. Green Chem. 2013, 15, 245–254. [Google Scholar] [CrossRef]
- Ferdosian, F.; Yuan, Z.; Anderson, M.; Xu, C. Synthesis of lignin-based epoxy resins: Optimization of reaction parame-ters using response surface methodology. RSC Adv. 2014, 4, 31745–31753. [Google Scholar] [CrossRef]
- Tanm, S.G.; Chow, W.S. Thermal properties of anhydride-cured bio-based epoxy blends. J. Therm. Anal. Calorim. 2010, 101, 1051–1058. [Google Scholar]
- Zhu, J.; Chandrashekhara, K.; Flanigan, V.; Kapila, J. Curing and mechanical characterization of a soy-based epoxy resin system. J. Appl. Polym. Sci. 2004, 91, 3513–3518. [Google Scholar] [CrossRef]
- Raquez, J.M.; Deleglise, M.; Lacrampe, M.F.; Krawczak, P. Thermosetting (bio)materials derived from renewable re-sources: A critical review. Prog. Polym. Sci. 2010, 35, 487–509. [Google Scholar] [CrossRef]
- Shi, X.H.; Chen, L.; Liu, B.W.; Long, J.W.; Xu, X.J.; Wang, Y.Z. Carbon fibers decorated by polyelectrolyte complexes toward their epoxy resin composites with high fire safety. Chin. J. Polym. Sci. 2018, 36, 1375–1384. [Google Scholar] [CrossRef]
- Jin, F.L.; Li, X.; Park, S.J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- vom Saal, F.S.; Hughes, C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 2005, 113, 926–933. [Google Scholar] [CrossRef] [Green Version]
- vom Saal, F.S.; Myers, J.P. Bisphenol A and risk of metabolic disorders. JAMA 2008, 300, 1353–1355. [Google Scholar] [CrossRef]
- Fache, M.; Darroman, E.; Besse, V.; Auvergne, R.; Caillol, S.; Boutevin, B. Vanillin, a promising biobased build-ing-block for monomer synthesis. Green Chem. 2014, 16, 1987–1998. [Google Scholar] [CrossRef]
- Fache, M.; Monteremal, C.; Boutevin, B.; Caillol, S. Amine hardeners and epoxy crosslinker from aromatic renewable resources. Eur. Polym. J. 2015, 73, 344–362. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin, a key-intermediate of biobased polymers. Eur. Polym. J. 2015, 68, 488–502. [Google Scholar] [CrossRef]
- Fache, M.; Auvergne, R.; Boutevin, B.; Caillol, S. New vanillin-derived diepoxy monomers for the synthesis of bi-obased thermosets. Eur. Polym. J. 2015, 67, 527–538. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Epoxy thermosets from model mixtures of the lignin-to-vanillin process. Green Chem. 2016, 18, 712–725. [Google Scholar] [CrossRef]
- Menard, R.; Negrell, C.; Fache, M.; Ferry, L.; Sonnier, R.; David, G. From a bio-based phosphorus-containing epoxy monomer to fully bio-based flame-retardant thermosets. RSC Adv. 2015, 5, 70856–70867. [Google Scholar] [CrossRef]
- Menard, R.; Negrell, C.; Ferry, L.; Sonnier, R.; David, G. Synthesis of biobased phosphorus-containing flame retardants for epoxy thermosets comparison of additive and reactive approaches. Polym. Degrad. Stab. 2016, 120, 300–312. [Google Scholar] [CrossRef]
- Fanny, J.; Hélène, N.; Bernard, B.; Sylvain, C. Synthesis of novel vinyl ester from biobased phloroglucinol. Green Mater. 2016, 4, 63–71. [Google Scholar]
- Aouf, C.; Nouailhas, H.; Fache, M.; Caillol, S.; Boutevin, B.; Fulcrand, B.H. Multifunctionalization of gallic acid. Synthesis of a novel bio-based epoxy resin. Eur. Polym. J. 2013, 49, 1185–1195. [Google Scholar]
- Nikafshar, S.; Zabihi, O.; Hamidi, S.; Moradi, Y.; Barzegar, S.; Ahmadi, M.; Naebe, M. A renewable bio-based epoxy resin with improved mechanical performance that can compete with DGEBA. RSC Adv. 2017, 7, 8694–8701. [Google Scholar] [CrossRef] [Green Version]
- Mauck, J.R.; Yadav, S.K.; Sadler, J.M.; La Scala, J.J.; Palmese, G.R.; Schmalbach, K.M.; Stanzione, J.F. Preparation and characterization of highly bio-based epoxy amine thermosets derived from lignocellulosics. Macromol. Chem. Phys. 2017, 218, 1700013. [Google Scholar] [CrossRef]
- Dean, J.M.; Grubbs, R.B.; Saad, W.; Cook, R.F.; Bates, F.S. Mechanical properties of block copolymer vesicle and micelle modified epoxies. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 2444–2456. [Google Scholar] [CrossRef]
- Rebizant, V.; Venet, A.-S.; Tournilhac, F.; Girard-Reydet, E.; Navarro, C.; Pascault, J.-P.; Leibler, L. Chemistry and mechanical properties of epoxy-based thermosets reinforced by reactive and nonreactive SBMX block copolymers. Macromolecules 2004, 34, 8017–8027. [Google Scholar] [CrossRef]
- Thio, Y.S.; Wu, J.; Bates, F.S. Epoxy toughening using low molecular weight poly(hexylene oxide)-poly(ethylene oxide) diblock copolymers. Macromolecules 2006, 39, 7187–7189. [Google Scholar] [CrossRef]
- Liu, J.D.; Thompson, Z.J.; Sue, H.-J.; Bates, F.S.; Hillmyer, M.A.; Dettloff, M.; Jacob, G.; Verghese, N.; Pham, H. Toughening of epoxies with block copolymer micelles of wormlike morphology. Macromolecules 2010, 43, 7238–7243. [Google Scholar] [CrossRef]
- Cano, L.; Builes, D.H.; Tercjak, A. Morphological and mechanical study of nanostructured epoxy systems modified with amphiphilic poly(ethylene oxide-b-propylene oxide-b-ethylene oxide)triblock copolymer. Polymer 2014, 55, 738–745. [Google Scholar] [CrossRef]
- Hillmyer, M.A.; Lipic, P.M.; Hajduk, D.A.; Almdal, K.; Bates, F.S. Self-assembly and polymerization of epoxy resin-amphiphilic block copolymer nanocomposites. J. Am. Chem. Soc. 1997, 119, 2749–2750. [Google Scholar] [CrossRef]
- Lipic, P.M.; Bates, F.S.; Hillmyer, M.A. Nanostructured thermosets from self-assembled amphiphilic block copolymer/epoxy resin mixtures. J. Am. Chem. Soc. 1998, 120, 8963–8970. [Google Scholar] [CrossRef]
- Mijovic, J.; Shen, M.; Sy, J.W.; Mondragon, I. Dynamics and morphology in nanostructured thermoset network/block copolymer blends during network formation. Macromolecules 2000, 33, 5235–5244. [Google Scholar] [CrossRef]
- Guo, Q.; Thomann, R.; Gronski, W.; Thurn-Albrecht, T. Phase behavior, crystallization, and hierarchical nanostructures in self-organized thermoset blends of epoxy resin and amphiphilic poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) triblock copolymers. Macromolecules 2002, 35, 3133–3144. [Google Scholar] [CrossRef]
- Sun, P.; Dang, Q.; Li, B.; Chen, T.; Wang, Y.; Lin, H.; Jin, Q.; Ding, D. Mobility, miscibility, and microdomain structure in nanostructured thermoset blends of epoxy resin and amphiphilic poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) triblock copolymers characterized by solid-state NMR. Macromolecules 2005, 38, 5654–5667. [Google Scholar] [CrossRef]
- Larrañaga, M.; Gabilondo, N.; Kortaberria, G.; Serrano, E.; Remiro, P.; Riccardi, C.C.; Mondragon, I. Micro- or nanoseparated phases in thermoset blends of an epoxy resin and PEO–PPO–PEO triblock copolymer. Polymer 2005, 46, 7082–7093. [Google Scholar] [CrossRef]
- Larrañaga, M.; Martin, M.D.; Gabilondo, N.; Kortaberria, G.; Eceiza, A.; Riccardi, C.C.; Mondragon, I. Toward microphase separation in epoxy systems containing PEO–PPO–PEO block copolymers by controlling cure conditions and molar ratios between blocks. Part 1. Cure kinetics. Colloid Polym. Sci. 2006, 284, 1403–1410. [Google Scholar] [CrossRef]
- Larrañaga, M.; Arruti, P.; Serrano, E.; de la Caba, K.; Remiro, P.; Riccardi, C.C.; Mondragon, I. Towards microphase separation in epoxy systems containing PEO/PPO/PEO block copolymers by controlling cure conditions and molar ratios between blocks. Part 2. Structural characterization. Colloid Polym. Sci. 2006, 284, 1419–1430. [Google Scholar] [CrossRef]
- Larrañaga, M.; Serrano, E.; Martin, M.D.; Tercjak, A.; Kortaberria, G.; de la Caba, K.; Riccardi, C.C.; Mondragon, I. Mechanical properties-morphology relationships in nano-/microstructured epoxy matrices modified with PEO-PPO-PEO block copolymers. Polym. Int. 2007, 56, 1392–1403. [Google Scholar] [CrossRef]
- Guo, Q.; Thomann, R.; Gronski, W.; Staneva, R.; Ivanova, R.; Stühn, B. Nanostructures, semicrytalline morphology, and nanoscale confinement effect on the crystallization kinetics in self-organized block copolymer/thermoset blends. Macromolecules 2003, 36, 3635–3645. [Google Scholar] [CrossRef]
- Tercjak, A.; Larrañaga, M.; Martin, M.D.; Mondragon, I. Thermally reversible nanostructured thermosetting blends modified with poly(ethylene-b-ethylene oxide) diblock copolymer. J. Therm. Anal. Calorim. 2006, 86, 663–668. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, F.; Wang, K.; Chen, L. Nanostructured thermoset epoxy resin templated by an amphiphilic poly(ethylene oxide)-block-poly(dimethylsiloxane) diblock copolymer. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 3042–3052. [Google Scholar] [CrossRef]
- Meng, F.; Zheng, S.; Liu, T. Epoxy resin containing poly(ethylene oxide)-block-poly(ε-caprolactone) diblock copolymer: Effect of curing agents on nanostructures. Polymer 2006, 47, 7590–7600. [Google Scholar] [CrossRef]
- Meng, F.; Zheng, S.; Li, H.; Liang, Q.; Liu, T. Formation of ordered nanostructures in epoxy thermosets: A mechanism of reaction-induced microphase separation. Macromolecules 2006, 39, 5072–5080. [Google Scholar] [CrossRef]
- Tercjak, A.; Serrano, E.; Garcia, I.; Mondragon, I. Thermoresponsive meso/nanostructured thermosetting materials based on PS-b-PEO block copolymer-dispersed liquid crystal: Curing behavior and morphological variation. Acta Mater. 2008, 56, 5112–5122. [Google Scholar] [CrossRef]
- Tercjak, A.; Mondragon, I. Relationships between the morphology and thermoresponsive behavior in micro/nanostructured thermosetting matrixes containing a 4′-(hexyloxy)-4-biphenylcarbonitrile liquid crystal. Langmuir 2008, 24, 11216–11224. [Google Scholar] [CrossRef]
- Tercjak, A.; Gutierrez, J.; Peponi, L.; Rueda, L.; Mondragon, I. Arrangement of conductive TiO2 nanoparticles in hybrid inorganic/organic thermosetting materials using liquid crystal. Macromolecules 2009, 42, 3386–3390. [Google Scholar] [CrossRef]
- Tercjak, A.; Gutierrez, J.; Mondragon, I. Conductive properties of inorganic/organic nanostructured systems based on block copolymers. Mater. Sci. Forum 2012, 714, 153–158. [Google Scholar] [CrossRef]
- Gutierrez, J.; Tercjak, A.; Mondragon, I. Transparent nanostructured thermoset composites containing well-dispersed TiO2 nanoparticles. J. Phys. Chem. C 2010, 114, 22424–22430. [Google Scholar] [CrossRef]
- Gutierrez, J.; Mondragon, I.; Tercjak, A. Morphological and optical behavior of thermoset matrix composites varying both polystyrene-block-poly(ethylene oxide) and TiO2 nanoparticle content. Polymer 2011, 52, 5699–5707. [Google Scholar] [CrossRef]
- Francis, R.; Baby, D.K. Toughening of epoxy thermoset with polystyrene-block-polyglycolic acid star copolymer: Nanostructure—Mechanical property correlation. Ind. Eng. Chem. Res. 2014, 53, 17945–17951. [Google Scholar] [CrossRef]
- Meng, F.; Zheng, S.; Zhang, W.; Li, H.; Liang, Q. Nanostructured thermosetting blends of epoxy resin and amphiphilic poly(ε-caprolactone)-block-polybutadiene-block-poly(ε-caprolactone) triblock copolymer. Macromolecules 2006, 39, 711–719. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, S. Reaction-induced microphase separation in epoxy thermosets containing poly(ε-caprolactone)-block-poly(n-butyl acrylate) diblock copolymer. Macromolecules 2007, 40, 2548–2558. [Google Scholar] [CrossRef]
- Ocando, C.; Serrano, E.; Tercjak, A.; Peña, C.; Kortaberria, G.; Calberg, C.; Grignard, B.; Jerome, R.; Carrasco, P.M.; Mecerreyes, D.; et al. Structure and properties of a semifluorinated diblock copolymer modified epoxy blend. Macromolecules 2007, 40, 4068–4074. [Google Scholar] [CrossRef]
- Fan, W.; Wang, L.; Zheng, S. Nanostructures in thermosetting blends of epoxy resin with polydimethylsiloxane-block-poly(ε-caprolactone)-block-polystyrene ABC triblock copolymer. Macromolecules 2009, 42, 327–336. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, C.; Han, J.; Zheng, S.; Li, X. Formation of nanophases in epoxy thermosets containing an organic–inorganic macrocyclic molecular brush with poly(ε-caprolactone)-block-polystyrene side chains. Soft Matter 2012, 8, 7062–7072. [Google Scholar] [CrossRef]
- Ji, S.; Wan, L.; Liu, C.C.; Nealey, P.F. Directed self-assembly of block copolymers on chemical patterns: A platform for nanofabrication. Prog. Polym. Sci. 2016, 54-55, 76–172. [Google Scholar] [CrossRef]
- Parameswaranpillai, J.; Sidhardhan, S.K.; Harikrishnan, P.; Pionteck, J.; Siengchin, S.; Unni, A.B.; Magueresse, A.; Grohens, Y.; Hameed, N.; Jose, S. Morphology, thermo-echanical properties and surface hydrofobicity of nanostructured epoxy thermosets modified with PEO-PPO-PEO triblock copolymer. Polym. Test. 2017, 59, 168–176. [Google Scholar] [CrossRef]
- Cherdoud-Chihani, A.; Mouzali, M.; Abadie, M.J.M. Study of crosslinking acid copolymer/DGEBA systems by FTIR. J. Appl. Polym. Sci. 2003, 87, 2033–2051. [Google Scholar] [CrossRef]
- Builes, D.H.; Hernandez, H.; Mondragon, I.; Tercjak, A. Relationship between the morphology of nanostructured unsaturated polyesters modified with PEO-b-PPO-b-PEO triblock copolymer and their optical and mechanical properties. J. Phys. Chem. C 2013, 117, 3563–3571. [Google Scholar] [CrossRef]








| BCPs | Abbreviation | Thermosetting Precursor | References |
|---|---|---|---|
| poly(hexylene oxide)-b-poly(ethylene oxide) | PHO-b-PEO | DGEBA + PN | [24] |
| poly(ethylene oxide)-b-poly(ethyl ethylene) | PEO-b-PEE | DGEBA + PA | [27] |
| poly(ethylene oxide)-b-poly(ethylene-alt-propylene) | PEO-b-PEP | DGEBA + MDA | [28] |
| poly(ethylene oxide)-b-poly(propylene oxide) | PEO-b-PPO | DGEBA + MDA | [29] |
| poly(ethylene oxide)-b-poly(propylene oxide)-b- poly(ethylene oxide) | PEO-b-PPO-b-PEO | DGEBA + MDA DGEBA + DDM | [30,31] [32,33,34,35] |
| polyethylene-b-poly(ethylene oxide) | PE-b-PEO | DGEBA + MDA DGEBA + MCDEA | [36] [37] |
| poly(ethylene oxide)-b-poly(dimethylsiloxane) | PEO-b-PDMS | DGEBA + MDA | [38] |
| poly(ethylene oxide)-b-poly(ε-caprolactone) | PEO-b-PCL | DGEBA + MOCA | [39] |
| poly(ethylene oxide)-b-polystyrene | PEO-b-PS | DGEBA + MDA DGEBA + MXDA DGEBA + MCDEA DGEBA + DDM | [40] [41,42,43,44] [45,46] [47] |
| poly(ε-caprolactone)-b-polybutadiene-b-poly(ε-caprolactone) | PCL-b-PB-b-PCL | DGEBA + MOCA | [48] |
| poly(ε-caprolactone)-b-poly(n-butyl acrylate) | PCL-b-PBA | DGEBA + MOCA | [49] |
| poly(heptadecafluorodecyl acrylate)-b-poly(caprolactone) | PaF-b-PCL | DGEBA + MCDEA | [50] |
| polydimethylsiloxane-b-poly(ε-caprolactone)-b-polystyrene | PDMS-b-PCL-b-PS | DGEBA + MOCA | [51] |
| poly(ε-caprolactone)-b-polystyrene | PCL-b-PS | DGEBA + MOCA | [52] |
| Material | Chemical Structure |
|---|---|
| DGEVA |  |
| DDM |  |
| PEO-PPO-PEO |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barandiaran, I.; Gomez-Hermoso-de-Mendoza, J.; Gutierrez, J.; Tercjak, A.; Kortaberria, G. Nanostructuring Biobased Epoxy Resin with PEO-PPO-PEO Block Copolymer. Polymers 2023, 15, 1216. https://doi.org/10.3390/polym15051216
Barandiaran I, Gomez-Hermoso-de-Mendoza J, Gutierrez J, Tercjak A, Kortaberria G. Nanostructuring Biobased Epoxy Resin with PEO-PPO-PEO Block Copolymer. Polymers. 2023; 15(5):1216. https://doi.org/10.3390/polym15051216
Chicago/Turabian StyleBarandiaran, Irati, Joseba Gomez-Hermoso-de-Mendoza, Junkal Gutierrez, Agnieszka Tercjak, and Galder Kortaberria. 2023. "Nanostructuring Biobased Epoxy Resin with PEO-PPO-PEO Block Copolymer" Polymers 15, no. 5: 1216. https://doi.org/10.3390/polym15051216
APA StyleBarandiaran, I., Gomez-Hermoso-de-Mendoza, J., Gutierrez, J., Tercjak, A., & Kortaberria, G. (2023). Nanostructuring Biobased Epoxy Resin with PEO-PPO-PEO Block Copolymer. Polymers, 15(5), 1216. https://doi.org/10.3390/polym15051216