Recent Advances in Centrifugal Spinning and Their Applications in Tissue Engineering
Abstract
:1. Introduction
2. Mechanism of Centrifugal Spinning
3. Factors Affecting Nanofiber Preparation
3.1. Polymer Melt or Solution Parameters
3.1.1. Concentration
3.1.2. Molecular Weight
3.1.3. Viscosity
3.1.4. Surface Tension
3.2. Machine Parameters
3.2.1. Flow Rate and Rotational Speed
3.2.2. Types of Collectors
3.2.3. Nozzle-Collector Spacing and Nozzle Geometry
3.2.4. Air Foil
3.3. Ambient Parameters
4. Types of Materials Used for Centrifugal Spinning
5. Fiber Characteristics
5.1. Fiber Homogeneity and Bead Formation
5.2. Fiber Alignment
5.3. Fiber Diameter Control
5.4. Fiber Porosity
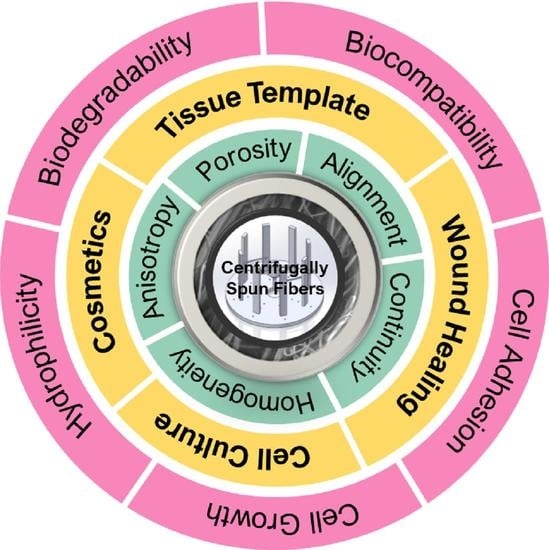
6. Nanocomposite Application for Tissue Engineering
6.1. Scaffold Fabrication
6.1.1. Tissue Template
6.1.2. Tissue-Wound Healing
6.2. Artificial Extracellular Matrix
6.3. 3D Cell Structure
6.4. Cosmetics
7. Challenges
8. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lannutti, J.; Reneker, D.; Ma, T.; Tomasko, D.; Farson, D. Electrospinning for tissue engineering scaffolds. Mater. Sci. Eng. C 2007, 27, 504–509. [Google Scholar] [CrossRef]
- Akia, M.; Capitanachi, D.; Martinez, M.; Hernandez, C.; de Santiago, H.; Mao, Y.; Lozano, K. Development and optimization of alumina fine fibers utilizing a centrifugal spinning process. Microporous Mesoporous Mater. 2018, 262, 175–181. [Google Scholar] [CrossRef]
- Padron, S.; Fuentes, A.; Caruntu, D.; Lozano, K. Experimental study of nanofiber production through forcespinning. J. Appl. Phys. 2013, 113, 024318. [Google Scholar] [CrossRef] [Green Version]
- Weng, B.; Xu, F.; Garza, G.; Alcoutlabi, M.; Salinas, A.; Lozano, K. The production of carbon nanotube reinforced poly (vinyl) butyral nanofibers by the Forcespinning® method. Polym. Eng. Sci. 2015, 55, 81–87. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Lu, Y.; Li, Y.; Zhang, X. SiO2/polyacrylonitrile membranes via centrifugal spinning as a separator for Li-ion batteries. J. Power Sources 2015, 273, 1114–1119. [Google Scholar] [CrossRef]
- Zhang, Z.-M.; Duan, Y.-S.; Xu, Q.; Zhang, B. A review on nanofiber fabrication with the effect of high-speed centrifugal force field. J. Eng. Fibers Fabr. 2019, 14, 1558925019867517. [Google Scholar] [CrossRef] [Green Version]
- Miele, D.; Nomicisio, C.; Musitelli, G.; Boselli, C.; Cornaglia, A.I.; Sànchez-Espejo, R.; Vigani, B.; Viseras, C.; Rossi, S.; Sandri, G. Design and development of polydioxanone scaffolds for skin tissue engineering manufactured via green process. Int. J. Pharm. 2023, 634, 122669. [Google Scholar] [CrossRef]
- Merchiers, J.; Martinez Narvaez, C.D.; Slykas, C.; Buntinx, M.; Deferme, W.; D’Haen, J.; Peeters, R.; Sharma, V.; Reddy, N.K. Centrifugally spun poly (ethylene oxide) fibers rival the properties of electrospun fibers. J. Polym. Sci. 2021, 59, 2754–2762. [Google Scholar] [CrossRef]
- Mahalingam, S.; Edirisinghe, M. Forming of polymer nanofibers by a pressurised gyration process. Macromol. Rapid Commun. 2013, 34, 1134–1139. [Google Scholar] [CrossRef] [Green Version]
- Badrossamay, M.R.; McIlwee, H.A.; Goss, J.A.; Parker, K.K. Nanofiber assembly by rotary jet-spinning. Nano Lett. 2010, 10, 2257–2261. [Google Scholar] [CrossRef] [Green Version]
- Rogalski, J.J.; Bastiaansen, C.W.; Peijs, T. PA6 nanofibre production: A comparison between rotary jet spinning and electrospinning. Fibers 2018, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Dotto, G.; Santos, J.; Tanabe, E.; Bertuol, D.; Foletto, E.; Lima, E.; Pavan, F. Chitosan/polyamide nanofibers prepared by Forcespinning® technology: A new adsorbent to remove anionic dyes from aqueous solutions. J. Clean. Prod. 2017, 144, 120–129. [Google Scholar] [CrossRef]
- Li, Y.; Zou, C.; Shao, J.; Zhang, X. Preparation of SiO2/PS superhydrophobic fibers with bionic controllable micro–nano structure via centrifugal spinning. RSC Adv. 2017, 7, 11041–11048. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.; Pandit, V.; Elkin, J.; Denman, T.; Cooper, J.A.; Kotha, S.P. Large-scale and highly efficient synthesis of micro-and nano-fibers with controlled fiber morphology by centrifugal jet spinning for tissue regeneration. Nanoscale 2013, 5, 2337–2345. [Google Scholar] [CrossRef] [PubMed]
- Sebe, I.; Szabó, B.; Nagy, Z.K.; Szabó, D.; Zsidai, L.; Kocsis, B.; Zelkó, R. Polymer structure and antimicrobial activity of polyvinylpyrrolidone-based iodine nanofibers prepared with high-speed rotary spinning technique. Int. J. Pharm. 2013, 458, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Mîndru, T.B.; Ignat, L.; Mîndru, I.B.; Pinteala, M. Morphological aspects of polymer fiber mats obtained by air flow rotary-jet spinning. Fibers Polym. 2013, 14, 1526–1534. [Google Scholar] [CrossRef]
- Abir, S.S.H.; Hasan, M.T.; Alcoutlabi, M.; Lozano, K. The effect of solvent and molecular weight on the morphology of centrifugally spun poly (vinylpyrrolidone) nanofibers. Fibers Polym. 2021, 22, 2394–2403. [Google Scholar] [CrossRef]
- Koski, A.; Yim, K.; Shivkumar, S. Effect of molecular weight on fibrous PVA produced by electrospinning. Mater. Lett. 2004, 58, 493–497. [Google Scholar] [CrossRef]
- Cremar, L.; Gutierrez, J.; Martinez, J.; Materon, L.; Gilkerson, R.; Xu, F.; Lozano, K. Development of antimicrobial chitosan based nanofiber dressings for wound healing applications. Nanomed. J. 2018, 5, 6–14. [Google Scholar]
- Zuniga, L.; Agubra, V.; Flores, D.; Campos, H.; Villareal, J.; Alcoutlabi, M. Multichannel hollow structure for improved electrochemical performance of TiO2/Carbon composite nanofibers as anodes for lithium ion batteries. J. Alloys Compd. 2016, 686, 733–743. [Google Scholar] [CrossRef]
- Padron, S.; Patlan, R.; Gutierrez, J.; Santos, N.; Eubanks, T.; Lozano, K. Production and characterization of hybrid BEH-PPV/PEO conjugated polymer nanofibers by forcespinning™. J. Appl. Polym. Sci. 2012, 125, 3610–3616. [Google Scholar] [CrossRef]
- Raghavan, B.; Soto, H.; Lozano, K. Fabrication of melt spun polypropylene nanofibers by forcespinning. J. Eng. Fibers Fabr. 2013, 8, 155892501300800106. [Google Scholar] [CrossRef] [Green Version]
- Zhiming, Z.; Boya, C.; Zilong, L.; Jiawei, W.; Yaoshuai, D. Spinning solution flow model in the nozzle and experimental study of nanofibers fabrication via high speed centrifugal spinning. Polymer 2020, 205, 122794. [Google Scholar] [CrossRef]
- Bano, S.; Akhtar, M.; Yasir, M.; Salman Maqbool, M.; Niaz, A.; Wadood, A.; Ur Rehman, M.A. Synthesis and characterization of silver–strontium (Ag-Sr)-doped mesoporous bioactive glass nanoparticles. Gels 2021, 7, 34. [Google Scholar] [CrossRef]
- Gonzalez, G.M.; MacQueen, L.A.; Lind, J.U.; Fitzgibbons, S.A.; Chantre, C.O.; Huggler, I.; Golecki, H.M.; Goss, J.A.; Parker, K.K. Production of synthetic, para-aramid and biopolymer nanofibers by immersion rotary jet-spinning. Macromol. Mater. Eng. 2017, 302, 1600365. [Google Scholar] [CrossRef]
- Badrossamay, M.R.; Balachandran, K.; Capulli, A.K.; Golecki, H.M.; Agarwal, A.; Goss, J.A.; Kim, H.; Shin, K.; Parker, K.K. Engineering hybrid polymer-protein super-aligned nanofibers via rotary jet spinning. Biomaterials 2014, 35, 3188–3197. [Google Scholar] [CrossRef] [Green Version]
- Zander, N.E. Formation of melt and solution spun polycaprolactone fibers by centrifugal spinning. J. Appl. Polym. Sci. 2015, 132, 41269. [Google Scholar] [CrossRef]
- Rane, Y.; Altecor, A.; Bell, N.S.; Lozano, K. Preparation of Superhydrophobic Teflon® AF 1600 Sub-Micron Fibers and Yarns Using the Forcespinningtm Technique. J. Eng. Fibers Fabr. 2013, 8, 155892501300800403. [Google Scholar] [CrossRef] [Green Version]
- Agubra, V.A.; De la Garza, D.; Gallegos, L.; Alcoutlabi, M. ForceSpinning of polyacrylonitrile for mass production of lithium-ion battery separators. J. Appl. Polym. Sci. 2016, 133, 42847. [Google Scholar] [CrossRef]
- Padilla-Gainza, V.; Rodríguez-Tobías, H.; Morales, G.; Ledezma-Pérez, A.; Alvarado-Canché, C.; Loera-Valencia, R.; Rodríguez, C.; Gilkerson, R.; De Leo, C.T.; Lozano, K. Development of zinc oxide/hydroxyapatite/poly (D, L-lactic acid) fibrous scaffold for tissue engineering applications. Biomater. Adv. 2022, 133, 112594. [Google Scholar] [CrossRef]
- Khang, A.; Ravishankar, P.; Krishnaswamy, A.; Anderson, P.K.; Cone, S.G.; Liu, Z.; Qian, X.; Balachandran, K. Engineering anisotropic biphasic Janus-type polymer nanofiber scaffold networks via centrifugal jet spinning. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2455–2464. [Google Scholar] [CrossRef]
- Zhou, H.; Tang, Y.; Wang, Z.; Zhang, P.; Zhu, Q. Cotton-like micro-and nanoscale poly (lactic acid) nonwoven fibers fabricated by centrifugal melt-spinning for tissue engineering. RSC Adv. 2018, 8, 5166–5179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rampichová, M.; Buzgo, M.; Chvojka, J.; Prosecká, E.; Kofroňová, O.; Amler, E. Cell penetration to nanofibrous scaffolds: Forcespinning®, an alternative approach for fabricating 3D nanofibers. Cell Adhes. Migr. 2014, 8, 36–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, E.; Ahmed, S.; Abir, S.S.H.; Taylor, K.; Padilla-Gainza, V.M.; Lozano, K. Centrifugally spun alginate-poly (lactic acid) microbeads: A promising carrier for drug delivery and tissue engineering. Int. J. Biol. Macromol. 2022, 220, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Tabata, Y. Effect of the fiber diameter and porosity of non-woven PET fabrics on the osteogenic differentiation of mesenchymal stem cells. J. Biomater. Sci. Polym. Ed. 2004, 15, 41–57. [Google Scholar] [CrossRef]
- Ghaderinejad, P.; Najmoddin, N.; Bagher, Z.; Saeed, M.; Karimi, S.; Simorgh, S.; Pezeshki-Modaress, M. An injectable anisotropic alginate hydrogel containing oriented fibers for nerve tissue engineering. Chem. Eng. J. 2021, 420, 130465. [Google Scholar] [CrossRef]
- Ravishankar, P.; Ozkizilcik, A.; Husain, A.; Balachandran, K. Anisotropic fiber-reinforced glycosaminoglycan hydrogels for heart valve tissue engineering. Tissue Eng. Part A 2021, 27, 513–525. [Google Scholar] [CrossRef]
- Janusz, W.; Skwarek, E. The Study of the Properties of the Hydroxyapatite/Electrolyte Interface. In Proceedings of the Annales Universitatis Mariae Curie-Sklodowska. 2009; p. 11. Available online: https://d1wqtxts1xzle7.cloudfront.net/77762885/tt_p-libre.pdf?1640923782=&response-content-disposition=inline%3B+filename%3DThe_study_of_the_properties_of_the_hydro.pdf&Expires=1677642347&Signature=OqjsJI3MewOOxTS1RUb2NgzaDkVlWIdxC~u9S3xd8aBuUgsjfje~niCrg3BXsAI3HJKIz2TPXxlGaijQ9DtcaPnqOArXULatrFoeBeYWm72ufseDZDcXHar7nwUyQty3vk4~62wYPIsPWCgpiWJvFyM0sCMBHpVk5dIYSSvuNGfHl7HRXb3Sg96v~h0zLS4Vdk5Hh4uDGz1qegTVBoc6z3YWc-uEIqN7stPH48FY9mURKapqIJjDC5NhEZLPJ-5AcoWncSn9AcyEdG9tuJHup2Iu-o77CezoWfGLDpaP7eJOVrG9z~JTmNXKv4Gk4wrXnXSSwhKPJlNEBYUPYcoTSg__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA (accessed on 20 January 2023).
- Akhtar, M.; Ahmed, S.; Hussain, R.; Wadood, A.; Roy, I.; Rehman, M.A.U.; Boccaccini, A.R. Centrifugal Spinning of Polyvinyl Alcohol/Sodium Alginate-Di-Aldehyde-Gelatin Based Antibacterial Nanofibers Intended for Skin Tissue Engineering. Mater. Lett. 2022, 323, 132530. [Google Scholar] [CrossRef]
- Lukášová, V.; Buzgo, M.; Vocetková, K.; Sovková, V.; Doupník, M.; Himawan, E.; Staffa, A.; Sedláček, R.; Chlup, H.; Rustichelli, F. Needleless electrospun and centrifugal spun poly-ε-caprolactone scaffolds as a carrier for platelets in tissue engineering applications: A comparative study with hMSCs. Mater. Sci. Eng. C 2019, 97, 567–575. [Google Scholar] [CrossRef]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Weng, B.; Materon, L.A.; Kuang, A.; Trujillo, J.A.; Lozano, K. Fabrication of cellulose fine fiber based membranes embedded with silver nanoparticles via Forcespinning. J. Polym. Eng. 2016, 36, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Rihova, M.; Lepcio, P.; Cicmancova, V.; Frumarova, B.; Hromadko, L.; Bureš, F.; Vojtova, L.; Macak, J.M. The centrifugal spinning of vitamin doped natural gum fibers for skin regeneration. Carbohydr. Polym. 2022, 294, 119792. [Google Scholar] [CrossRef]
- Eftekhari-Pournigjeh, F.; Saeed, M.; Rajabi, S.; Tamimi, M.; Pezeshki-Modaress, M. Three-dimensional biomimetic reinforced chitosan/gelatin composite scaffolds containing PLA nano/microfibers for soft tissue engineering application. Int. J. Biol. Macromol. 2023, 225, 1028–1037. [Google Scholar] [CrossRef]
- Liu, M.; Lin, Z.; Wang, X.; Yan, W. Focused rotary jet spinning: A novel fiber technology for heart biofabrication. Matter 2022, 5, 3576–3579. [Google Scholar] [CrossRef]
- Rogalski, J.J.; Bastiaansen, C.W.; Peijs, T. Rotary jet spinning review–a potential high yield future for polymer nanofibers. Nanocomposites 2017, 3, 97–121. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Yu, Z.; Li, C.; Lv, Y.; Hong, S.; Hu, P.; Liu, Y. Review of the principles, devices, parameters, and applications for centrifugal electrospinning. Macromol. Mater. Eng. 2022, 307, 2200057. [Google Scholar] [CrossRef]
- Atıcı, B.; Ünlü, C.H.; Yanilmaz, M. A review on centrifugally spun fibers and their applications. Polym. Rev. 2022, 62, 1–64. [Google Scholar] [CrossRef]
- Zannini Luz, H.; Loureiro dos Santos, L.A. Centrifugal spinning for biomedical use: A review. Crit. Rev. Solid State Mater. Sci. 2022, 1–16. [Google Scholar] [CrossRef]
- Xu, H.; Yagi, S.; Ashour, S.; Du, L.; Hoque, M.E.; Tan, L. A Review on Current Nanofiber Technologies: Electrospinning, Centrifugal Spinning, and Electro-Centrifugal Spinning. Macromol. Mater. Eng. 2022, 2200502. [Google Scholar] [CrossRef]
- Sarkar, K.; Gomez, C.; Zambrano, S.; Ramirez, M.; de Hoyos, E.; Vasquez, H.; Lozano, K. Electrospinning to forcespinning™. Mater. Today 2010, 13, 12–14. [Google Scholar] [CrossRef]
- Shanmuganathan, K.; Fang, Y.; Chou, D.Y.; Sparks, S.; Hibbert, J.; Ellison, C.J. Solventless high throughput manufacturing of poly (butylene terephthalate) nanofibers. ACS Macro Lett. 2012, 1, 960–964. [Google Scholar] [CrossRef]
- Edmondson, D.; Cooper, A.; Jana, S.; Wood, D.; Zhang, M. Centrifugal electrospinning of highly aligned polymer nanofibers over a large area. J. Mater. Chem. 2012, 22, 18646–18652. [Google Scholar] [CrossRef]
- Hufenus, R.; Yan, Y.; Dauner, M.; Kikutani, T. Melt-spun fibers for textile applications. Materials 2020, 13, 4298. [Google Scholar] [CrossRef]
- Riahi, D.N. Nonlinear rotating viscoelastic jets during forcespinning process. Proc. R. Soc. A 2018, 474, 20180346. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Sun, J.; Shao, M.; Yang, B. A comparison of centrifugally-spun and electrospun regenerated silk fibroin nanofiber structures and properties. Rsc Adv. 2015, 5, 98553–98558. [Google Scholar] [CrossRef]
- Hashemi, A.R.; Pishevar, A.R.; Valipouri, A.; Părău, E.I. Numerical and experimental study on the steady cone-jet mode of electro-centrifugal spinning. Phys. Fluids 2018, 30, 017103. [Google Scholar] [CrossRef] [Green Version]
- Sinatra, N.R.; Lind, J.U.; Parker, K.K. Fabricating multi-material nanofabrics using rotary jet spinning. In Proceedings of the 2017 IEEE 17th International Conference on Nanotechnology (IEEE-NANO), Pittsburgh, PA, USA, 25–28 July 2017; pp. 715–719. [Google Scholar]
- Andjani, D.; Sriyanti, I.; Fauzi, A.; Edikresnha, D.; Munir, M.M. Fabrication of polyvinylpyrrolidone fibers by Means of Rotary Forcespinning Method. IOP Conf. Ser. Mater. Sci. Eng. 2018, 367, 012044. [Google Scholar] [CrossRef]
- Chen, B.; Wang, J.; Lai, Z.; Zhang, Z.; Wu, Z. Modeling of spinning jet behavior and evaluation on fiber morphology for centrifugal spinning. J. Text. Inst. 2022, 113, 1438–1449. [Google Scholar] [CrossRef]
- Engström, J.; Hagström, B. Centrifugal spinning of nanofiber webs: A parameter study of a novel spinning process. Nord. Text. J. 2009. [Google Scholar]
- Li, X.; Chen, H.; Yang, B. Centrifugally spun starch-based fibers from amylopectin rich starches. Carbohydr. Polym. 2016, 137, 459–465. [Google Scholar] [CrossRef]
- Akia, M.; Cremar, L.; Seas, M.; Villarreal, J.; Valdez, A.; Alcoutlabi, M.; Lozano, K. High-Throughput Production With Improved Functionality and Graphitization of Carbon Fine Fibers Developed from Sodium Chloride-Polyacrylonitrile Precursors. Polym. Eng. Sci. 2018, 58, 2047–2054. [Google Scholar] [CrossRef]
- Vo, P.P.; Doan, H.N.; Kinashi, K.; Sakai, W.; Tsutsumi, N.; Huynh, D.P. Centrifugally spun recycled PET: Processing and characterization. Polymers 2018, 10, 680. [Google Scholar] [CrossRef] [Green Version]
- Szabó, P.; Kállai-Szabó, B.; Kállai-Szabó, N.; Sebe, I.; Zelkó, R. Preparation of hydroxypropyl cellulose microfibers by high-speed rotary spinning and prediction of the fiber-forming properties of hydroxypropyl cellulose gels by texture analysis. Cellulose 2014, 21, 4419–4427. [Google Scholar] [CrossRef]
- Golecki, H.M.; Yuan, H.; Glavin, C.; Potter, B.; Badrossamay, M.R.; Goss, J.A.; Phillips, M.D.; Parker, K.K. Effect of solvent evaporation on fiber morphology in rotary jet spinning. Langmuir 2014, 30, 13369–13374. [Google Scholar] [CrossRef] [Green Version]
- Merchiers, J.; Martínez Narváez, C.D.; Slykas, C.; Reddy, N.K.; Sharma, V. Evaporation and rheology chart the processability map for centrifugal force spinning. Macromolecules 2021, 54, 11061–11073. [Google Scholar] [CrossRef]
- Schabikowski, M.; Tomaszewska, J.; Kata, D.; Graule, T. Rotary jet-spinning of hematite fibers. Text. Res. J. 2015, 85, 316–324. [Google Scholar] [CrossRef]
- Stojanovska, E.; Kurtulus, M.; Abdelgawad, A.; Candan, Z.; Kilic, A. Developing lignin-based bio-nanofibers by centrifugal spinning technique. Int. J. Biol. Macromol. 2018, 113, 98–105. [Google Scholar] [CrossRef]
- Ren, L.; Kotha, S.P. Centrifugal jet spinning for highly efficient and large-scale fabrication of barium titanate nanofibers. Mater. Lett. 2014, 117, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Wallwork, I.; Decent, S.; King, A.; Schulkes, R. The trajectory and stability of a spiralling liquid jet. Part 1. Inviscid theory. J. Fluid Mech. 2002, 459, 43–65. [Google Scholar] [CrossRef]
- Decent, S.; King, A.; Wallwork, I. Free jets spun from a prilling tower. J. Eng. Math. 2002, 42, 265–282. [Google Scholar] [CrossRef]
- Akia, M.; Cremar, L.; Chipara, M.; Munoz, E.; Cortez, H.; De Santiago, H.; Rodriguez-Macias, F.J.; Vega-Cantú, Y.I.; Arandiyan, H.; Sun, H. In Situ production of graphene–fiber hybrid structures. ACS Appl. Mater. Interfaces 2017, 9, 25474–25480. [Google Scholar] [CrossRef]
- Loordhuswamy, A.M.; Krishnaswamy, V.R.; Korrapati, P.S.; Thinakaran, S.; Rengaswami, G.D.V. Fabrication of highly aligned fibrous scaffolds for tissue regeneration by centrifugal spinning technology. Mater. Sci. Eng. C 2014, 42, 799–807. [Google Scholar] [CrossRef]
- Upson, S.J.; O’Haire, T.; Russell, S.J.; Dalgarno, K.; Ferreira, A.M. Centrifugally spun PHBV micro and nanofibres. Mater. Sci. Eng. C 2017, 76, 190–195. [Google Scholar] [CrossRef]
- McEachin, Z.; Lozano, K. Production and characterization of polycaprolactone nanofibers via forcespinning™ technology. J. Appl. Polym. Sci. 2012, 126, 473–479. [Google Scholar] [CrossRef]
- Xu, F.; Weng, B.; Materon, L.A.; Gilkerson, R.; Lozano, K. Large-scale production of a ternary composite nanofiber membrane for wound dressing applications. J. Bioact. Compat. Polym. 2014, 29, 646–660. [Google Scholar] [CrossRef]
- Li, Q.; Lozano, K.; Lü, Y.; Mao, Y. Heterogeneous Manganese Oxide-Encased Carbon Nanocomposite Fibers for High Performance Pseudocapacitors. Ceram. Mater. Energy Appl. III 2013, 34, 41–55. [Google Scholar]
- Potter, G.; Barbosa, R.; Villarreal, A.; Salinas, A.; Guzman, H.; De Leon, H.; Ortega, J.A.; Lozano, K. Design and Validation of a Portable Handheld Device to Produce Fine Fibers Using Centrifugal Forces. Instruments 2020, 4, 27. [Google Scholar] [CrossRef]
- Jao, D.; Lima, T.A.; Thursch, L.; Flamini, M.D.; Pressly, J.; Ippolito, J.; Alvarez, N.J.; Beachley, V. Highly Aligned Centrifugal Spun Polyacrylonitrile Nanofibers Collected and Processed with Automated Tracks. Macromol. Mater. Eng. 2022, 308, 2200488. [Google Scholar] [CrossRef]
- Ren, L. Centrifugal Jet Spinning of Polymer Nanofiber Assembly: Process Characterization and Engineering Applications; Rensselaer Polytechnic Institute: New York, NY, USA, 2014. [Google Scholar]
- Sebe, I.; Kállai-Szabó, B.; Kovács, K.N.; Szabadi, E.; Zelkó, R. Micro-and macrostructural characterization of polyvinylpirrolidone rotary-spun fibers. Drug Dev. Ind. Pharm. 2015, 41, 1829–1834. [Google Scholar] [CrossRef]
- Akia, M.; Mkhoyan, K.A.; Lozano, K. Synthesis of multiwall α-Fe2O3 hollow fibers via a centrifugal spinning technique. Mater. Sci. Eng. C 2019, 102, 552–557. [Google Scholar] [CrossRef]
- Hou, T.; Li, X.; Lu, Y.; Yang, B. Highly porous fibers prepared by centrifugal spinning. Mater. Des. 2017, 114, 303–311. [Google Scholar] [CrossRef]
- Mihut, D.M.; Lozano, K.; Foltz, H. Fabrication and characterization of silver-and copper-coated Nylon 6 forcespun nanofibers by thermal evaporation. J. Vac. Sci. Technol. A Vac. Surf. Film. 2014, 32, 061401. [Google Scholar] [CrossRef] [Green Version]
- Akia, M.; Salinas, N.; Rodriguez, C.; Gilkerson, R.; Materon, L.; Lozano, K. Texas sour orange juice used in scaffolds for tissue engineering. Membranes 2018, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Boyle, W.S.; Chen, W.; Rodriguez, A.; Linn, S.; Tolar, J.; Lozano, K.; Reineke, T.M. Ternary composite nanofibers containing chondroitin sulfate scavenge inflammatory chemokines from solution and prohibit squamous cell carcinoma migration. ACS Appl. Bio Mater. 2019, 2, 619–624. [Google Scholar] [CrossRef]
- Vida, T.A.; Motta, A.C.; Santos, A.R.; Cardoso, G.B.C.; Brito, C.C.d.; Zavaglia, C.A.d.C. Fibrous PCL/PLLA scaffolds obtained by rotary jet spinning and electrospinning. Mater. Res. 2018, 20, 910–916. [Google Scholar] [CrossRef]
- Heseltine, P.L.; Bayram, C.; Gultekinoglu, M.; Homer-Vanniasinkam, S.; Ulubayram, K.; Edirisinghe, M. Facile One-Pot Method for All Aqueous Green Formation of Biocompatible Silk Fibroin-Poly (Ethylene Oxide) Fibers for Use in Tissue Engineering. ACS Biomater. Sci. Eng. 2022, 8, 1290–1300. [Google Scholar] [CrossRef]
- O’Haire, T.; Russell, S.; Carr, C.M. Centrifugal melt spinning of polyvinylpyrrolidone (PVP)/triacontene copolymer fibres. J. Mater. Sci. 2016, 51, 7512–7522. [Google Scholar] [CrossRef] [Green Version]
- Bortolotto Degregori, E.; Corbellini Henckes, N.A.; Franco, N.; Luz, H.; Maurmann, N.; Viana, A.R.; Rohden, F.; Loureiro dos Santos, L.A.; Cirne Lima, E.O.; Terraciano, P.B. Interaction between adipoderivated mesenchymal stem cells and PLGA/PI epox scaffold with possible use in tissue engineering: In vitro study. Int. J. Polym. Mater. Polym. Biomater. 2022, 1–10. [Google Scholar] [CrossRef]
- Ren, L.; Ozisik, R.; Kotha, S.P. Rapid and efficient fabrication of multilevel structured silica micro-/nanofibers by centrifugal jet spinning. J. Colloid Interface Sci. 2014, 425, 136–142. [Google Scholar] [CrossRef]
- Weng, B.; Xu, F.; Salinas, A.; Lozano, K. Mass production of carbon nanotube reinforced poly (methyl methacrylate) nonwoven nanofiber mats. Carbon 2014, 75, 217–226. [Google Scholar] [CrossRef]
- Vazquez, B.; Vasquez, H.; Lozano, K. Preparation and characterization of polyvinylidene fluoride nanofibrous membranes by forcespinning™. Polym. Eng. Sci. 2012, 52, 2260–2265. [Google Scholar] [CrossRef]
- Krifa, M.; Hammami, M.A.; Wu, H. Occurrence and morphology of bead-on-string structures in centrifugal forcespun PA6 fibers. J. Text. Inst. 2015, 106, 284–294. [Google Scholar] [CrossRef]
- Ayati, S.S.; Karevan, M.; Stefanek, E.; Bhia, M.; Akbari, M. Nanofibers Fabrication by Blown-Centrifugal Spinning. Macromol. Mater. Eng. 2022, 307, 2100368. [Google Scholar] [CrossRef]
- Grosberg, A.; Kuo, P.-L.; Guo, C.-L.; Geisse, N.A.; Bray, M.-A.; Adams, W.J.; Sheehy, S.P.; Parker, K.K. Self-organization of muscle cell structure and function. PLoS Comput. Biol. 2011, 7, e1001088. [Google Scholar] [CrossRef]
- Atıcı, B.; Ünlü, C.H.; Yanilmaz, M. A statistical analysis on the influence of process and solution properties on centrifugally spun nanofiber morphology. J. Ind. Text. 2022, 51, 613S–639S. [Google Scholar] [CrossRef]
- Xia, L.; Feng, H.; Zhang, Q.; Luo, X.; Fei, P.; Li, F. Centrifugal spinning of lignin amine/cellulose acetate nanofiber for heavy metal ion adsorption. Fibers Polym. 2022, 23, 77–85. [Google Scholar] [CrossRef]
- Gholipour-Kanani, A.; Daneshi, P. A Review on Centrifugal and Electro-Centrifugal Spinning as New Methods of Nanofibers Fabrication. J. Text. Polym. 2022, 10, 41–55. [Google Scholar]
- Patlan, R.; Mejias, J.; McEachin, Z.; Salinas, A.; Lozano, K. Fabrication and characterization of poly (L-lactic acid) fiber mats using centrifugal spinning. Fibers Polym. 2018, 19, 1271–1277. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Hou, T.; Zhou, J.; Wang, A.; Zhang, X.; Yang, B. Jet evolution and fiber formation mechanism of amylopectin rich starches in centrifugal spinning system. J. Appl. Polym. Sci. 2021, 138, 50275. [Google Scholar] [CrossRef]
- Fang, Y.; Herbert, M.; Schiraldi, D.A.; Ellison, C.J. Tin fluorophosphate nonwovens by melt state centrifugal Forcespinning. J. Mater. Sci. 2014, 49, 8252–8260. [Google Scholar] [CrossRef]
- Pai, C.-L.; Boyce, M.C.; Rutledge, G.C. Morphology of porous and wrinkled fibers of polystyrene electrospun from dimethylformamide. Macromolecules 2009, 42, 2102–2114. [Google Scholar] [CrossRef]
- Ma, M.; Gupta, M.; Li, Z.; Zhai, L.; Gleason, K.K.; Cohen, R.E.; Rubner, M.F.; Rutledge, G.C. Decorated electrospun fibers exhibiting superhydrophobicity. Adv. Mater. 2007, 19, 255–259. [Google Scholar] [CrossRef]
- Agubra, V.A.; Zuniga, L.; De la Garza, D.; Gallegos, L.; Pokhrel, M.; Alcoutlabi, M. Forcespinning: A new method for the mass production of Sn/C composite nanofiber anodes for lithium ion batteries. Solid State Ion. 2016, 286, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Arican, F.; Uzuner-Demir, A.; Polat, O.; Sancakli, A.; Ismar, E. Fabrication of gelatin nanofiber webs via centrifugal spinning for N95 respiratory filters. Bull. Mater. Sci. 2022, 45, 93. [Google Scholar] [CrossRef]
- Padilla-Gainza, V.M.; Rodríguez-Tobías, H.; Morales, G.; Saucedo-Salazar, E.; Lozano, K.; Montaño-Machado, V.; Mantovani, D. Centrifugally spun mats based on biopolyesters/hydroxyapatite and their potential as bone scaffolds. J. Appl. Polym. Sci. 2021, 138, app50139. [Google Scholar] [CrossRef]
- Eslami, M.; Vrana, N.E.; Zorlutuna, P.; Sant, S.; Jung, S.; Masoumi, N.; Khavari-Nejad, R.A.; Javadi, G.; Khademhosseini, A. Fiber-reinforced hydrogel scaffolds for heart valve tissue engineering. J. Biomater. Appl. 2014, 29, 399–410. [Google Scholar] [CrossRef]
- Jang, J.; Lee, J.; Seol, Y.-J.; Jeong, Y.H.; Cho, D.-W. Improving mechanical properties of alginate hydrogel by reinforcement with ethanol treated polycaprolactone nanofibers. Compos. Part B Eng. 2013, 45, 1216–1221. [Google Scholar] [CrossRef]
- Villarreal-Gómez, L.J.; Vera-Graziano, R.; Vega-Ríos, M.R.; Pineda-Camacho, J.L.; Almanza-Reyes, H.; Mier-Maldonado, P.A.; Cornejo-Bravo, J.M. Biocompatibility evaluation of electrospun scaffolds of poly (L-Lactide) with pure and grafted hydroxyapatite. J. Mex. Chem. Soc. 2014, 58, 435–443. [Google Scholar]
- Felice, B.; Sánchez, M.A.; Socci, M.C.; Sappia, L.D.; Gómez, M.I.; Cruz, M.K.; Felice, C.J.; Martí, M.; Pividori, M.I.; Simonelli, G. Controlled degradability of PCL-ZnO nanofibrous scaffolds for bone tissue engineering and their antibacterial activity. Mater. Sci. Eng. C 2018, 93, 724–738. [Google Scholar] [CrossRef] [Green Version]
- Zhong, S.; Teo, W.E.; Zhu, X.; Beuerman, R.W.; Ramakrishna, S.; Yung, L.Y.L. An aligned nanofibrous collagen scaffold by electrospinning and its effects on in vitro fibroblast culture. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2006, 79, 456–463. [Google Scholar] [CrossRef]
- Mary, L.A.; Senthilram, T.; Suganya, S.; Nagarajan, L.; Venugopal, J.; Ramakrishna, S.; Giri Dev, V. Centrifugal spun ultrafine fibrous web as a potential drug delivery vehicle. Express Polym. Lett. 2013, 7, 238–248. [Google Scholar] [CrossRef]
- Norzain, N.A.; Lin, W.-C.; Razali, N.A.M. Triangular-prism Microstructure Engineered on the Fibrous Scaffold Using Electro-centrifugal Spinning Technique for Tissue Engineering. Fibers Polym. 2022, 23, 3398–3414. [Google Scholar] [CrossRef]
- Hasan, M.T.; Gonzalez, R.; Chipara, M.; Materon, L.; Parsons, J.; Alcoutlabi, M. Antibacterial activities of centrifugally spun polyethylene oxide/silver composite nanofibers. Polym. Adv. Technol. 2021, 32, 2327–2338. [Google Scholar] [CrossRef]
- Ahn, S.; Chantre, C.O.; Gannon, A.R.; Lind, J.U.; Campbell, P.H.; Grevesse, T.; O’Connor, B.B.; Parker, K.K. Soy protein/cellulose nanofiber scaffolds mimicking skin extracellular matrix for enhanced wound healing. Adv. Healthc. Mater. 2018, 7, 1701175. [Google Scholar] [CrossRef] [PubMed]
- Marginean Lazar, G.; Fructus, A.; Baillet, A.; Bocquet, J.; Thomas, P.; Marty, J. Sunscreens’ photochemical behaviour: In vivo evaluation by the stripping method. Int. J. Cosmet. Sci. 1997, 19, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Priyanto, A.; Hapidin, D.A.; Khairurrijal, K. Potential Loading of Virgin Coconut Oil into Centrifugally-Spun Nanofibers for Biomedical Applications. ChemBioEng Rev. 2022, 9, 393–408. [Google Scholar] [CrossRef]
- Loreana, M.; Luissanyi, C.; Luis, S.; Yuanbing, M.; Nancy, F. Development and Characterization of Glandless Cottonseed Meal/Pullulan Fine Fiber Mats. Arch. Nanomed. 2018, 1, 117. [Google Scholar]
- Bao, N.; Wei, Z.; Ma, Z.; Liu, F.; Yin, G. Si-doped mesoporous TiO2 continuous fibers: Preparation by centrifugal spinning and photocatalytic properties. J. Hazard. Mater. 2010, 174, 129–136. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Pei, S.; Liu, G.; Liu, J. Preparation of nanocrystalline titanium dioxide fibers using sol–gel method and centrifugal spinning. J. Sol-Gel Sci. Technol. 2013, 65, 443–451. [Google Scholar] [CrossRef]














| Polymers (P) | Solvent (S) | Molecular Weight (Mw) of Polymer | Rotational Speed (rpm) | Average Diameter (nm) | Weight Percentage, wt% (P/S) | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Polylactic acid (PLA) | Chloroform | - | 4000/8000/12,000 | 1143/468/424 | 8/NA | Tissue engineering scaffolds | [28] |
| Hydroxypropyl cellulose | Distilled water | 80,000 | 7 × 103 to 20 × 103 | 48 | Formulation process of pharmaceutically active compound | [63] | |
| Polyvinylpyrrolidone (PVP), Kollidon 30 | Mixture of water & Ethanol (1:1) | 30,000–50,000 | - | (11 ± 3.7) ×103 | 45/NA | conventional dosage forms or drug delivery systems | [82] |
| Polyvinylpyrrolidone (PVP) | Ethanol | 1,300,000 | 15,000 | (4.85 ± 1.37) × 103 (16) | 8, 10, 12, 14, 16, 18 | Actives ingredients in drug delivery system | [56] |
| PVP/Iron Nitrate | Water | 1,300,000 | 7000–7500 | 852 ± 86 (OD) | - | Catalyst, biological processes and biomedical applications | [83] |
| Ethyl cellulose (EC)/PVP | Ethanol & Water | NA/1300,000 | 3500 | 7000 | - | Tissue engineering, drug delivery | [84] |
| Tannic acid (TA)/PVA/Chitosan (CS) | Distilled water | 85,000–124,000/50,000–190,000 | 20,000 | 800 | 10 | Wound dressing | [76] |
| PVA, Alginate di-aldehyde (ADA)/PVA | Deionized water | - | 3000 | 139 ± 62, 258 ± 42 | 10 | Skin tissue engineering | [36] |
| Polycaprolactone (PCL) | 1,1,1,3,3,3-Hexafluoro-2-propanol (HFIP) | 70,000–90,000 | 30,000 | - | 6 (w/v) | Tissue engineering | [21] |
| PCL | Dichloromethane, DMF, Chloroform | 60,000, 80,000 | 9000, 4000 | 220 ± 98, 990 | 16,18 | Neural tissue regeneration and tissue engineering | [33,75] |
| Nylon 6/Polyurethane (PU) | Dimethylformamide & Acetone | - | 30,000 | - | 5/5 | Flexible sensors, and drug-eluting materials | [55] |
| Nylon 6/Ag-Cu NPs | Formic Acid | - | 8000 | ≥100 | - | Wound dressing, tissue engineering | [85] |
| Polybutylene terephthalate (PBT) | Polymer Melt | - | 10,000, 12,000, 15,000 | 1350, 1310, 1380 | - | High-performance polymer fiber with desirable chemical/physical properties | [49] |
| Polyhydroxy butyrate (PHB) | Chloroform | 550,000 | 5000–6000 | 1800 | 11 | Tissue engineering | [86] |
| Chitosan (CS) | Trifluoroacetic acid & DCM | - | 6000–9000 | 800 to 1500 | 7–9 | Wound dressing | [13] |
| Teflon-AF (TAF) 1600 | Fluorinert FC-40 | - | 5000–10,000 | 362 ± 58 | 10 | Protective agent | [24] |
| Polystyrene (PS) | Dimethylformamide | 260,000 | 5000–8000 | 9000, 5500, 3300, 2000 | 16, 18, 20, 22, 24 | Superhydrophobic surface | [6] |
| Pullulan (PL)/Chondroitin Sulphate | Tannic acid (TA) & Citric acid (CA) | - | 6500 | 400 | 18 | Wound dressing | [87] |
| Poly(L-lactic acid) (PLLA)/PCL | Chloroform, Chloroform/Acetone | 177,500/70,000–90,000 | 3450 | 1647 ± 976, 8002 ± 5051 | 6 | Scaffold for cell growth | [88] |
| PU, Gelatin/PU | DMF | 100,000 | 2800–3500 | 100 to 700, 2000 to 12,000 | 20 | Dressing, scaffolding | [10] |
| Poly (vinyl chloride (PVC) | Cyclohexanon & DMF | 230,000 | 3000 | 2000 to 12,000 | 16 | Dressing, scaffolding | [10] |
| Lignin/Thermoplastic polyurethane (TPU) | DMF | - | 6000–11,000 | <500 | 15, 20, 25 | Biomedical applications | [67] |
| Poly (3-hydroxybutyrate-co-3-hydroxyvalerate)(PHBV) | Chloroform | - | 9000 | 500 to 3000 | 25 | Biomedical applications | [73] |
| PLA melt | - | 90,595 | 900 | 3470 to 3480 | - | Tissue engineering | [29] |
| Chitosan/gelatin | Chloroform, DMF | 190,000–310,000 | 10,000 | 1810 | 30 | Soft tissue engineering | [41] |
| Poly(D,L-lactic aid)/ZnO/hydroxyapatite | Chloroform | 160,000 | 400–600 | 780 ± 550 to 2390 ± 740 | 10 | Bone tissue engineering | [26] |
| Silk fibroin (SF)/poly(ethylene oxide) (PEO) | Lithium bromide (for SF), Deionized water (for PEO) | 200,000 (PEO) | 36,000 | 710 to 2100 | 15 (PEO) | Bone tissue engineering | [89] |
| Poly (ethylene oxide) | Water | 600,000 | 10,000 | 447 ± 165 to 596 ± 222 | - | Cosmetic and dermatologic application | [40] |
| Polyvinylpyrrolidone/1-triacontene | - | - | 7000–15,000 | 1000 to 2000 | - | Adsorbent in cosmetic application | [90] |
| Polylactic-co-glycolic acid (PLGA) | Chloroform | - | - | - | 1.5 | Tissue engineering | [91] |
| Polydioxanone (PDO) | Deep eutectic solvents (DES) | - | 700 | 10,000 to 20,000 | - | Wound healing | [4] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marjuban, S.M.H.; Rahman, M.; Duza, S.S.; Ahmed, M.B.; Patel, D.K.; Rahman, M.S.; Lozano, K. Recent Advances in Centrifugal Spinning and Their Applications in Tissue Engineering. Polymers 2023, 15, 1253. https://doi.org/10.3390/polym15051253
Marjuban SMH, Rahman M, Duza SS, Ahmed MB, Patel DK, Rahman MS, Lozano K. Recent Advances in Centrifugal Spinning and Their Applications in Tissue Engineering. Polymers. 2023; 15(5):1253. https://doi.org/10.3390/polym15051253
Chicago/Turabian StyleMarjuban, Shaik Merkatur Hakim, Musfira Rahman, Syeda Sharmin Duza, Mohammad Boshir Ahmed, Dinesh K. Patel, Md Saifur Rahman, and Karen Lozano. 2023. "Recent Advances in Centrifugal Spinning and Their Applications in Tissue Engineering" Polymers 15, no. 5: 1253. https://doi.org/10.3390/polym15051253
APA StyleMarjuban, S. M. H., Rahman, M., Duza, S. S., Ahmed, M. B., Patel, D. K., Rahman, M. S., & Lozano, K. (2023). Recent Advances in Centrifugal Spinning and Their Applications in Tissue Engineering. Polymers, 15(5), 1253. https://doi.org/10.3390/polym15051253