Polysaccharides Are Effective Inhibitors of Natural Gas Hydrate Formation
Abstract
:1. Introduction
- -
- At the water (ice)–gas interface;
- -
- In the volume of free gas saturated with water vapor;
- -
- In the volume of gas-saturated water;
- -
- In the volume of the gas-saturated oil fluid.
2. Formation of Gas Hydrates in Oil and Gas Production Processes
3. Prevention and Control of Gas Hydrate Formation in Oil and Gas Production Processes
- -
- Thermodynamic hydrate inhibitors, whose action is based on the shift the hydrate-liquid-vapor equilibrium of gas hydrate formation towards lower temperatures and high pressures (methanol, ethanol, ethylene glycol, glycols, salt solutions, etc.);
- -
- Kinetic hydrate inhibitors (KHIs), which are water-soluble polymers that prevent or delay the nucleation and/or growth of hydrates (homo- and copolymers of N-vinylcaprolactam, N-isopropylacrylamide, and N-vinylpyrrolidone);
- -
- Anti-agglomerates (AAs), which are surfactants that do not stop nucleation but stop the agglomeration (sticking together) of gas hydrate crystals.
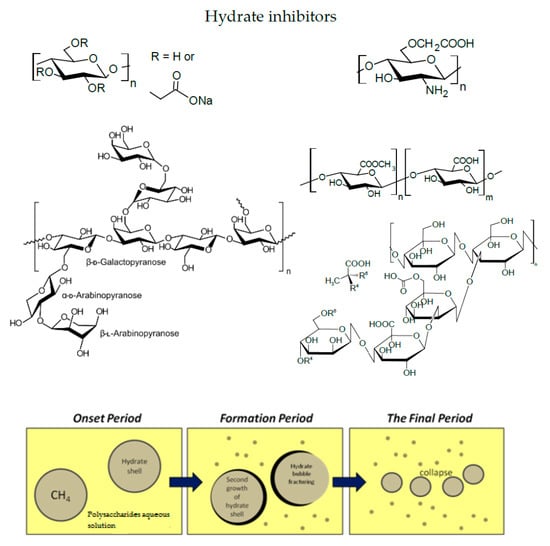
4. Polysaccharides Are Promising “Green” Inhibitors of Gas Hydrate Formation
5. Practical Aspects of the Use of Polysaccharides in Inhibiting Hydrate Formation
- -
- According to the corrosion aggressiveness of commercial mold, the corrosion rate of carbon steel at 20 °C is 0.0042 g/(m2·h);
- -
- The inhibitory effect exceeds the effectiveness of methanol;
- -
- The solidification temperature is −51 °C;
- -
- The kinematic viscosity is 17.7 mm2/s at −40 °C;
6. Conclusions
- -
- functionalization of polysaccharides (introduction of carboxyl, amide and ether fragments);
- -
- increasing the degree of branching of the main chain of polysaccharides;
- -
- search for synergistic additives to polysaccharides and the creation on their basis of new highly effective inhibitors of hydrate formation, economically feasible for industrial use;
- -
- search for the optimal molecular weight of polysaccharides for use as inhibitors of gas hydrate formation.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Barker, J.W.; Gomez, R.K. Formation of Hydrates during Deepwater Drilling Operations. J. Pet. Technol. 1989, 41, 297–301. [Google Scholar] [CrossRef]
- Mu, L.; von Solms, N. Inhibition of natural gas hydrate in the system containing salts and crude oil. J. Pet. Sci. Eng. 2020, 188, 106940. [Google Scholar] [CrossRef]
- Birchwood, R.; Dai, J.; Shelander, D.; Boswell, R. Developments in Gas Hydrates. Oilfield Rev. 2010, 22, 18–33. [Google Scholar]
- Creek, J.L. Efficient Hydrate Plug Prevention. Energy Fuels 2012, 26, 4112–4116. [Google Scholar] [CrossRef]
- Sloan, E.D. Natural Gas Hydrates in Flow Assurance; Gulf Professional Publishing: Oxford, UK, 2010. [Google Scholar]
- Englezos, P.; Huang, Z.; Bishnoi, P.R. Prediction of Natural Gas Hydrate Formation Conditions in the Presence of Methanol Using the Trebble-Bishnoi Equation of State. J. Can. Pet. Technol. 1991, 30, 149–155. [Google Scholar] [CrossRef]
- Sloan, E.D. A Changing Hydrate Paradigm—From Apprehension to Avoidance to Risk Management. Fluid Ph. Equilibria 2005, 228–229, 67–74. [Google Scholar] [CrossRef]
- Boxall, J.; Davies, S.; Koh, C.; Sloan, E.D. Predicting When and Where Hydrate Plugs Form in Oil-dominated Flowlines. SPE Proj. Facil. Constr. 2009, 4, 80–86. [Google Scholar] [CrossRef]
- Phillips, N.J.; Grainger, M. Development and Application of Kinetic Hydrate Inhibitors in the North Sea. In Proceedings of the SPE Gas Technology Symposium, Calgary, AB, Canada, 15–18 March 1998. [Google Scholar] [CrossRef]
- Giavarini, C.; Hester, K. Gas Hydrates Immense Energy Potential and Environmental Challenges, 1st ed.; Springer: London, UK, 2011. [Google Scholar]
- Makogon, T.Y. Handbook of Multiphase Flow Assurance; Gulf Professional Publishing: Cambridge, MA, USA, 2019; pp. 95–189. [Google Scholar] [CrossRef]
- Chazallon, B.; Rodriguez, C.T.; Ruffine, L.; Carpentier, Y.; Donval, J.-P.; Ker, S.; Riboulot, V. Characterizing the variability of natural gas hydrate composition from a selected site of the Western Black Sea, off Romania. Mar. Pet. Geol. 2021, 124, 104785. [Google Scholar] [CrossRef]
- Shao, J.; Wang, Y.; Wang, Y.; Yan, H. High-Resolution Seismic Characterization of Gas Hydrate Reservoir Using Wave-Equation-Based Inversion. Energies 2022, 15, 7652. [Google Scholar] [CrossRef]
- Boswell, R.; Collett, T.S. Current perspectives on gas hydrate resources. Energy Environ. Sci. 2011, 4, 1206–1215. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Carroll, J. Natural Gas Hydrates. A Guide for Engineers; Gulf Professional Publishing: Oxford, UK, 2014. [Google Scholar]
- Manakov, A.Y.; Stoporev, A.S. Physical chemistry and technological applications of gas hydrates: Topical aspects. Russ. Chem. Rev. 2021, 90, 566–600. [Google Scholar] [CrossRef]
- Fu, J.; Mo, J.-M.; Liu, S.-J.; Yi, W.-Z.; Yu, Y.-S.; Wu, N.-Y.; Chen, X.-L.; Su, Q.-C.; Li, X.-S. Thermodynamic characteristics of methane hydrate formation in high-pressure microcalorimeter under different reaction kinetics. Fuel 2023, 332, 126072. [Google Scholar] [CrossRef]
- Ke, W.; Svartaas, T.M.; Chen, D. A Review of Gas Hydrate Nucleation Theories and Growth Models. J. Nat. Gas Eng. 2018, 61, 169–196. [Google Scholar] [CrossRef]
- Mohammad, B.; Tehrani, D.M. Effect of magnetic field on gas hydrate formation. Nat. Gas Ind. B 2022, 9, 240–245. [Google Scholar] [CrossRef]
- Kvamme, B.; Wei, N.; Zhao, J.; Zhou, S.; Zhang, L.; Sun, W.; Saeidi, N. Routes to hydrate formation from water dissolved in gas and impact of mineral surfaces. Petroleum 2021, 7, 385–401. [Google Scholar] [CrossRef]
- Almashwali, A.A.; Bavoh, C.B.; Lal, B.; Khor, S.F.; Jin, Q.C.; Zaini, D. Gas Hydrate in Oil-Dominant Systems: A Review. ACS Omega 2022, 7, 27021–27037. [Google Scholar] [CrossRef]
- Feder, J. Study Correlates Hydrate Blockage Risk and Gas/Liquid Flow Pattern in Horizontal Pipelines. J. Pet. Technol. 2020, 72, 45–46. [Google Scholar] [CrossRef]
- Jujuly, M.M.; Rahman, M.A.; Maynard, A.; Adey, M. Hydrate-Induced Vibration in an Offshore Pipeline. SPE J. 2020, 25, 732–743. [Google Scholar] [CrossRef]
- Kakitani, C.; Marques, D.C.; Neto, M.A.M.; Teixeira, A.; Valim, L.S.; Morales, R.E.M.; Sum, A.K. Dynamics of Hydrate Behavior in Shut-In and Restart Condition in Two and Three Phase System. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 4–7 May 2020. [Google Scholar] [CrossRef]
- Lau, H.C.; Zhang, M.; Wang, J.; Pan, L. Some Technical Considerations of Gas-Hydrate Development from Chinese Permafrost Regions. SPE Reserv. Eval. Eng. 2020, 23, 369–387. [Google Scholar] [CrossRef]
- Saeed, Z.; Emamzadeh, A. Modelling the Formation of Gas Hydrate in the Pipelines. Pet. Petro. Chem. Eng. J. 2021, 5, 000259. [Google Scholar] [CrossRef]
- Pickarts, M.A.; Delgado-Linares, J.; Brown, E.; Veedu, V.; Koh, C.A. Evaluation of a Robust, In-Situ Surface Treatment for Pipeline Solids Deposition Mitigation in Flowing Systems. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 4–7 May 2020. [Google Scholar] [CrossRef]
- Soliman Sahweity, M.A. Hydrate Management Controls in Saudi Aramco’s Largest Offshore Nonassociated Gas Fields. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 4–7 May 2020. [Google Scholar] [CrossRef]
- Srivastava, V.; Majid, A.A.A.; Warrier, P.; Grasso, G.; Koh, C.A.; Zerpa, L.E. Hydrate-Bedding Mechanisms in Partially Dispersed Water/Oil Systems. SPE J. 2020, 25, 0925–0937. [Google Scholar] [CrossRef]
- Vu, V.Q.; Suchaux, P.D.; Fürst, W. Use of a predictive electrolyte equation of state for the calculation of the gas hydrate formation temperature in the case of systems with methanol and salts. Fluid Ph. Equilibria 2002, 194–197, 361–370. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Joonaki, E.; Vasheghani Farahani, M.; Takeya, S.; Ruppel, C.; Yang, J.; English, N.J.; Schicks, J.M.; Edlmann, K.; Mehrabian, H.; et al. Gas hydrates in sustainable chemistry. Chem. Soc. Rev. 2020, 49, 5225–5309. [Google Scholar] [CrossRef] [PubMed]
- Sayani, J.K.; Yadavalli, S.S.; Mamidi, L.T.; Kamireddi, V.R. Investigation on the Kinetic Behavior of Gas Hydrates Based on Induction Time for a High CO2 Mixed Gas Multiphase Pipeline System. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 9–12 November 2020. [Google Scholar] [CrossRef]
- Kumar, A.; Di Lorenzo, M.; Kozielski, K.; Singh, A.; May, E.F.; Aman, Z.M. Hydrate Management in Restart Operations of a Subsea Jumper. In Proceedings of the Offshore Technology Conference Asia, Kuala Lumpur, Malaysia, 3–6 November 2020. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Tong, S.; Gong, Z.; Ma, N.; Sun, B. Hydrate Plugging Prevention in Deep Water Gas Wells. In Proceedings of the 8th SPE/AAPG/SEG Unconventional Resources Technology Conference, Virtual, 20–22 July 2020. [Google Scholar] [CrossRef]
- Kwak, G.H.; Lee, K.H.; Lee, B.R.; Sum, A.K. Quantification of the risk for hydrate formation during cool down in a dispersed oil-water system. Korean J. Chem. Eng. 2017, 34, 2043–2048. [Google Scholar] [CrossRef]
- Shi, B.-H.; Song, S.-F.; Lv, X.-F.; Li, W.-Q.; Wang, Y.; Ding, L.; Liu, Y.; Yang, J.-H.; Wu, H.-H.; Wang, W.; et al. Investigation on natural gas hydrate dissociation from a slurry to a water-in-oil emulsion in a high-pressure flow loop. Fuel 2018, 233, 743–758. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, B.; Liu, Y.; Song, S.; Gong, J. Experimental and Theoretical Investigation of the Interaction between Hydrate Formation and Wax Precipitation in Water-in-Oil Emulsions. Energy Fuels 2018, 32, 9081–9092. [Google Scholar] [CrossRef]
- Daraboina, N.; Pachitsas, S.; von Solms, N. Natural gas hydrate formation and inhibition in gas/crude oil/aqueous systems. Fuel 2015, 148, 86–190. [Google Scholar] [CrossRef]
- Stoporev, A.S.; Manakov, A.Y.; Altunina, L.K.; Bogoslovskii, A.V.; Strelets, L.A.; Aladko, E.Y. Dependence of the rate of formation and the P-T stability field of methane hydrate suspensions in crude oils upon oil composition. Pet. Chem. 2014, 54, 171–176. [Google Scholar] [CrossRef]
- Sloan, E.D.; Fleyfel, F. A molecular mechanism for gas hydrate nucleation from ice. AIChE J. 1991, 37, 1281–1292. [Google Scholar] [CrossRef]
- Khurana, M.; Yin, Z.; Linga, P. A Review of Clathrate Hydrate Nucleation. ACS Sustain. Chem. Eng. 2017, 5, 11176–11203. [Google Scholar] [CrossRef]
- Yin, Z.; Khurana, M.; Tan, H.K.; Linga, P. A review of gas hydrate growth kinetic models. Chem. Eng. J. 2018, 342, 9–29. [Google Scholar] [CrossRef]
- Persiyantsev, M.N. Extraction of Oil under Complicated Conditions; Nedra-Biznestsentr: Moscow, Russia, 2000. [Google Scholar]
- Abegunde, M.; Adeyemi, A. Optimisation of Thermal Insulation of Subsea Flowlines for Hydrates Prevention. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Virtual, 11–13 August 2020. [Google Scholar] [CrossRef]
- Santos, H.F.L.; Perondi, E.A.; Wentz, A.V.; Silva Júnior, A.L.; Barone, D.A.C.; Galassi, M.; Castro, B.B.D.; Ferreira, A.M.G.; dos Reis, N.R.S.; Pereira Pinto, H.L.D.C.; et al. Proposal and Experimental Trials on a Robot for Hydrate and Paraffin Removal in Submarine Flexible Lines. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 4–7 May 2020. [Google Scholar] [CrossRef]
- Larsen, R.; Knight, C.A.; Sloan, E.D. Clathrate Hydrate Growth and Inhibition. Fluid Ph. Equilibria 1998, 150–151, 353–360. [Google Scholar] [CrossRef]
- Kelland, M.A. Production Chemicals for the Oil and Gas Industry; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Lal, B.; Nashed, O. Chemical Additives for Gas Hydrates. Green Energy and Technology; Springer: New York, NY, USA, 2020. [Google Scholar] [CrossRef]
- Kamal, M.S.; Hussein, I.A.; Sultan, A.S.; von Solms, N. Application of various water soluble polymers in gas hydrate inhibition. Renew. Sustain. Energy Rev. 2016, 60, 206–225. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Kelland, M.A.; Lu, H. Non-amide kinetic hydrate inhibitors: A review. Fuel 2022, 315, 123179. [Google Scholar] [CrossRef]
- Nosov, V.V.; Voloshin, A.I.; Dokichev, V.A. Ingibitory gazogidratoobrazovaniya: Nastoyashchee i budushchee [Inhibitors of Gas Hydrate Formation: Present and Future]. Probl. Gather. Treat. Transp. Oil Oil Prod. 2022, 5, 58–72. [Google Scholar] [CrossRef]
- Yaqub, S.; Murtaza, M.; Lal, B. Towards a fundamental understanding of biopolymers and their role in gas hydrates: A review. J. Nat. Gas Eng. 2021, 91, 103892. [Google Scholar] [CrossRef]
- Singh, A.; Suri, A. A review on gas hydrates and kinetic hydrate inhibitors based on acrylamides. J. Nat. Gas Eng. 2020, 83, 103539. [Google Scholar] [CrossRef]
- Farhadian, A.; Shadloo, A.; Zhao, X.; Pavelyev, R.S.; Peyvandi, K.; Qiu, Z.; Varfolomeev, M.A. Challenges and advantages of using environmentally friendly kinetic gas hydrate inhibitors for flow assurance application: A comprehensive review. Fuel 2023, 336, 127055. [Google Scholar] [CrossRef]
- Elhenawy, S.; Khraisheh, M.; Almomani, F.; Al-Ghouti, M.A.; Hassan, M.K.; Al-Muhtaseb, A. Towards Gas Hydrate-Free Pipelines: A Comprehensive Review of Gas Hydrate Inhibition Techniques. Energies 2022, 15, 8551. [Google Scholar] [CrossRef]
- Kelland, M.A. History of the Development of Low Dosage Hydrate Inhibitors. Energy Fuels 2006, 20, 825–847. [Google Scholar] [CrossRef]
- Kelland, M.A. Designing Kinetic Hydrate Inhibitors—Eight Projects with Only Partial Success, But Some Lessons Learnt. Energy Fuels 2017, 31, 5046–5054. [Google Scholar] [CrossRef]
- Kelland, M.A. A Review of Kinetic Hydrate Inhibitors from an Environmental Perspective. Energy Fuels 2018, 32, 12001–12012. [Google Scholar] [CrossRef]
- Kelland, M.A. Challenges with gas hydrate formation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 700, 012057. [Google Scholar] [CrossRef] [Green Version]
- Cha, M.; Shin, K.; Seo, Y.; Shin, J.-Y.; Kang, S.-P. Catastrophic Growth of Gas Hydrates in the Presence of Kinetic Hydrate Inhibitors. J. Phys. Chem. A 2013, 117, 13988–13995. [Google Scholar] [CrossRef]
- Dokichev, V.A.; Voloshin, A.I.; Nifantiev, N.E.; Egorov, M.P.; Kireeva, D.R.; Isakov, A.V.; Bakhtizin, R.N.; Rabaev, R.U. New “Green” Inhibitors of Gas Hydrate Formation for the Oil and Gas Industry Based on Polysaccharides. SOCAR Proc. 2021, 1, 33–40. [Google Scholar] [CrossRef]
- Voloshin, A.; Nifantiev, N.; Egorov, M.; Alimbekov, R.; Dokichev, V. Development and Implementation of Green Inhibitors of Gas Hydrate Formation in the Fields of Western Siberia. In Proceedings of the SPE Russian Petroleum Technology Conference, Virtual, 12–15 October 2021. [Google Scholar] [CrossRef]
- Mitchell, G.F.; Talley, L.D. Application of Kinetic Hydrate Inhibitor in Black-Oil Flowlines. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 3–6 October 1999. [Google Scholar] [CrossRef]
- Fu, S.B.; Cenegy, L.M.; Neff, C.S. A Summary of Successful Field Applications of a Kinetic Hydrate Inhibitor. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 13–16 February 2001. [Google Scholar] [CrossRef]
- Budd, D.; Hurd, D.; Pakulski, M.; Schaffer, T.D. Enhanced Hydrate Inhibition in Alberta Gas Field. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 26–29 September 2004. [Google Scholar] [CrossRef]
- Swanson, T.A.; Petrie, M.; Sifferman, T.R. The Successful Use of Both Kinetic Hydrate and Paraffin Inhibitors Together in a Deepwater Pipeline with a High Water Cut in the Gulf of Mexico. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 2–4 February 2005. [Google Scholar] [CrossRef]
- Farhadian, A.; Varfolomeev, M.A.; Kudbanov, A.; Rezaeisadat, M.; Nurgaliev, D.K. Waterborne polymers as kinetic/anti-agglomerant methane hydrate and corrosion inhibitors: A new and promising strategy for flow assurance. J. Nat. Gas Eng. 2020, 77, 103235. [Google Scholar] [CrossRef]
- Ke, W.; Chen, D. A short review on natural gas hydrate, kinetic hydrate inhibitors and inhibitor synergists. Chin. J. Chem. Eng. 2019, 27, 2049–2061. [Google Scholar] [CrossRef]
- Carpenter, C. Benefits of Low-Dosage Hydrate Inhibitors. J. Pet. Technol. 2019, 71, 94–95. [Google Scholar] [CrossRef]
- Perrin, A.; Musa, O.M.; Steed, J.W. The chemistry of low dosage clathrate hydrate inhibitors. Chem. Soc. Rev. 2013, 42, 1996–2015. [Google Scholar] [CrossRef] [Green Version]
- Arjmandi, M.; Tohidi, B.; Danesh, A.; Todd, A.C. Is Subcooling the Right Driving Force for Testing Low Dosage Hydrate Inhibitors? Chem. Eng. Sci. 2005, 60, 1313–1321. [Google Scholar] [CrossRef]
- Colle, K.S.; Talley, L.D.; Longo, J.M. A Method for Inhibiting Hydrate Formation. WO 2005/005567 A1, 15 June 2004. [Google Scholar]
- Maeda, N.; Fong, C.; Sheng, Q.; da Silveira, K.C.; Tian, W.; Seeber, A.; Ganther, W.; Kelland, M.A.; Mady, M.F.; Wood, C.D. High-Throughput Testing of Kinetic Hydrate Inhibitors. Energy Fuels 2016, 30, 5432–5438. [Google Scholar] [CrossRef]
- Sun, M.; Firoozabadi, A. Gas hydrate powder formation—Ultimate solution in natural gas flow assurance. Fuel 2015, 146, 1–5. [Google Scholar] [CrossRef]
- Kelland, M.A.; Svartaas, T.M.; Øvsthus, J.; Tomita, T.; Chosa, J.-i. Studies on some zwitterionic surfactant gas hydrate anti-agglomerants. Chem. Eng. Sci. 2006, 61, 4048–4059. [Google Scholar] [CrossRef]
- Meshram, S.B.; Sardar, H.; Kushwaha, O.S.; Sangwai, J.; Kumar, R. A systematic molecular investigation on Sodium Dodecyl Benzene Sulphonate (SDBS) as a Low Dosage Hydrate Inhibitor (LDHI) and the role of Benzene Ring in the structure. J. Mol. Liq. 2021, 337, 116374. [Google Scholar] [CrossRef]
- Gupta, P.; Sangwai, J.S. Formation and dissociation kinetics of methane hydrate in aqueous oilfield polymer solutions (polyacrylamide, xanthan gum, and guar gum) and their performance evaluation as low-dosage kinetic hydrate inhibitors (LDHI). Energy Fuels 2019, 33, 6335–6349. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, N.; Liang, D.-Q. Inhibition effects of polysaccharides for gas hydrate formation in methane–water system. J. Mol. Liq. 2019, 292, 111435. [Google Scholar] [CrossRef]
- Farhadian, A.; Varfolomeev, M.A.; Shaabani, A.; Nasiri, S.; Vakhitov, I.; Zaripova, Y.F.; Yarkovoi, V.V.; Sukhov, A.V. Sulfonated Chitosan as Green and High Cloud Point Kinetic Methane Hydrate and Corrosion Inhibitor: Experimental and Theoretical Studies. Carbohydr. Polym. 2020, 236, 116035. [Google Scholar] [CrossRef] [PubMed]
- Altamash, T.; Qureshi, M.F.; Aparicio, S.; Aminnaji, M.; Tohidi, B.; Atilhan, M. Gas hydrates inhibition via combined biomolecules and synergistic materials at wide process conditions. J. Nat. Gas Eng. 2017, 46, 873–883. [Google Scholar] [CrossRef]
- Ghosh, R.; Kelland, M.A. Nonpolymeric Citramide-Based Kinetic Hydrate Inhibitors: Good Performance with Just Six Alkylamide Groups. ACS Omega 2022, 7, 13953–13962. [Google Scholar] [CrossRef]
- Farhadian, A.; Varfolomeev, M.A.; Rahimi, A.; Mendgaziev, R.I.; Semenov, A.P.; Stoporev, A.S.; Vinogradova, S.S.; Karwt, R.; Kelland, M.A. Gas Hydrate and Corrosion Inhibition Performance of the Newly Synthesized Polyurethanes: Potential Dual Function Inhibitors. Energy Fuels 2021, 35, 6113–6124. [Google Scholar] [CrossRef]
- Sanatgar, S.M.; Peyvandi, K. New edible additives as Green Inhibitors for Preventing Methane Hydrate Formation. J. Environ. Chem. Eng. 2019, 7, 103172. [Google Scholar] [CrossRef]
- Farhadian, A.; Varfolomeev, M.A.; Shaabani, A.; Zaripova, Y.F.; Yarkovoi, V.V.; Khayarov, K.R. Inhibition Performance of Chitosan-graft-Polyacrylamide as an Environmentally Friendly and High-Cloud-Point Inhibitor of Nucleation and Growth of Methane Hydrate. Cryst. Growth Des. 2020, 20, 1771–1778. [Google Scholar] [CrossRef]
- Semenov, A.P.; Mendgaziev, R.I.; Stoporev, A.S.; Kuchierskaya, A.A.; Novikov, A.A.; Vinokurov, V.A. Gas Hydrate Nucleation and Growth in the Presence of Water-soluble Polymer, Nonionic Surfactants, and Their Mixtures. J. Nat. Gas Eng. 2020, 82, 103491. [Google Scholar] [CrossRef]
- Tang, C.; Liang, D. Inhibitory effects of novel green inhibitors on gas hydrate formation. Chin. J. Chem. Eng. 2019, 27, 2107–2117. [Google Scholar] [CrossRef]
- Farhadian, A.; Varfolomeev, M.A.; Kudbanov, A.; Gallyamov, S.R. A new class of promising biodegradable kinetic/anti-agglomerant methane hydrate inhibitors based on castor oil. Chem. Eng. Sci. 2019, 206, 507–512. [Google Scholar] [CrossRef]
- Pavelyev, R.S.; Zaripova, Y.F.; Yarkovoi, V.V.; Vinogradova, S.S.; Razhabov, S.; Khayarov, K.R.; Nazarychev, S.A.; Stoporev, A.S.; Mendgaziev, R.I.; Semenov, A.P.; et al. Performance of Waterborne Polyurethanes in Inhibition of Gas Hydrate Formation and Corrosion: Influence of Hydrophobic Fragments. Molecules 2020, 25, 5664. [Google Scholar] [CrossRef]
- Tohidi, B.; Anderson, R.; Mozaffar, H.; Tohidi, F. The Return of Kinetic Hydrate Inhibitors. Energy Fuels 2015, 29, 8254–8260. [Google Scholar] [CrossRef]
- Nasir, Q.; Suleman, H.; Elsheikh, Y.A. Review on the role and impact of various additives as promoters/ inhibitors for gas hydrate formation. J. Nat. Gas. Sci. Eng. 2020, 76, 103211. [Google Scholar] [CrossRef]
- Cohen, J.M.; Wolf, P.F.; Young, W.D. Enhanced Hydrate Inhibitors: Powerful Synergism with Glycol Ethers. Energy Fuels 1998, 12, 216–218. [Google Scholar] [CrossRef]
- Hussain, H.H.; Husin, H. The effect of synergistic amino acids-ionic liquids in methane hydrate inhibition by COSMO-RS application. J. Mol. Liq. 2021, 321, 114837. [Google Scholar] [CrossRef]
- Rossi, F.; Gambelli, A.M. Thermodynamic phase equilibrium of single-guest hydrate and formation data of hydrate in presence of chemical additives: A review. Fluid Ph. Equilibria 2021, 536, 112958. [Google Scholar] [CrossRef]
- Kim, J.; Shin, K.; Seo, Y.; Cho, S.J.; Lee, J.D. Synergistic Hydrate Inhibition of Monoethylene Glycol with Poly(vinylcaprolactam) in Thermodynamically Underinhibited System. J. Phys. Chem. B 2014, 118, 9065–9075. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-Y.; Feng, J.-C.; Ke, W.; Wang, J.; Zhang, S.; Xie, Y. Film formation kinetics of Methane-propane hydrate on gas bubble in MEG and luvicap EG solutions. Appl. Energy 2023, 330, 120301. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Shen, Y.; Cheng, L.; Liu, B.; Yan, K.; Chen, G.; Li, T. Molecular dynamics simulation to explore the synergistic inhibition effect of kinetic and thermodynamic hydrate inhibitors. Energy 2022, 238, 121697. [Google Scholar] [CrossRef]
- Saberi, A.; Alamdari, A.; Rasoolzadeh, A.; Mohammadi, A.H. Insights into Kinetic Inhibition Effects of MEG, PVP, and L-Tyrosine Aqueous Solutions on Natural Gas Hydrate Formation. Pet. Sci. 2021, 18, 495–508. [Google Scholar] [CrossRef]
- Finch, P. Carbohydrates. Structures, Synthesis and Dynamics; Springer: Dordrecht, The Netherlands, 1999. [Google Scholar]
- Abbaszadeh, A.; Lad, M.; Janin, M.; Morris, G.A.; MacNaughtan, W.; Sworn, G.; Foster, T.J. A novel approach to the determination of the pyruvate and acetate distribution in xanthan. Food Hydrocoll. 2015, 44, 162–171. [Google Scholar] [CrossRef]
- Guenet, M.J. Polymer-Solvent Molecular Compounds; Elsevier Ltd.: Oxford, UK, 2008. [Google Scholar]
- Garcia-Ochoa, F.; Santos Mazorra, V.E.; Casas, J.A.; Gomez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of xanthan gum as polysaccharide in tissue engineering: A review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef]
- Nazarzadeh Zare, E.; Makvandi, P.; Tay, F.R. Recent progress in the industrial and biomedical applications of tragacanth gum. Carbohydr. Polym. 2019, 212, 450–467. [Google Scholar] [CrossRef]
- Tamang, N.; Shrestha, P.; Khadka, B.; Mondal, M.H.; Saha, B.; Bhattarai, A. A Review of Biopolymers’ Utility as Emulsion Stabilizers. Polymers 2022, 14, 127. [Google Scholar] [CrossRef]
- OSPAR List of Substances Used and Discharged Offshore Which Are Considered to Pose Little or No Risk to the Environment (PLONOR); OSPAR Agreement 2012-06 (Replacing Agreement 2004-10); OSPAR Commission: London, UK, 2013.
- Baraka-Lokmane, S.; Sorbie, K.; Poisson, N.; Kohler, N. Can green scale inhibitors replace phosphonate scale inhibitors? Carbonate coreflooding experiments. Pet. Sci. Technol. 2009, 27, 427–441. [Google Scholar] [CrossRef]
- Teptereva, G.A.; Pahomov, S.I.; Chetvertneva, I.A.; Karimov, J.H.; Egorov, M.P.; Movsumzade, J.M.; Evstigneev, J.I.; Vasil’ev, A.V.; Sevast’janova, M.V.; Voloshin, A.I.; et al. Renewable natural raw materials. structure, properties, application prospects. Chem. Chem. Tech. 2021, 64, 4–121. [Google Scholar] [CrossRef]
- Voloshin, A.I.; Gusakov, V.N.; Fakhreeva, A.V.; Dokichev, V.A. Scaling prevention inhibitors in oil production. Oilfield Eng. 2018, 11, 60–72. [Google Scholar] [CrossRef]
- Safronov, A.P.; Adamova, L.V.; Kurlyandskaya, G.V. Flory–huggins parameters of guar gum, xanthan gum, agarose, and gellan gum in aqueous solutions. Polym. Sci.-A 2019, 61, 29–38. [Google Scholar] [CrossRef]
- Yaqub, S.; Lal, B.; Shariff, A.b.M.; Mellon, N.B. Unraveling the Effect of Sub-Cooling Temperatures on the Kinetic Performance of Biopolymers for Methane Hydrate. J. Nat. Gas Sci. Eng. 2019, 65, 68–81. [Google Scholar] [CrossRef]
- Singh, A.; Suri, A. Enhanced Hydrate Inhibition by Plant-Based Polysaccharides as Synergists with Kinetic Hydrate Inhibitors. Energy Fuels 2022, 36, 6974–6988. [Google Scholar] [CrossRef]
- Suri, A.; Singh, A. Synergistic Hydrate Inhibition by Iota-Carrageenan with Kinetic Hydrate Inhibitors. In Proceedings of the Middle East Oil, Gas and Geosciences Show, Manama, Bahrain, 19–21 February 2023. SPE-213610-MS. [Google Scholar] [CrossRef]
- Yaqub, S.; Lal, B.; Keong, L.K. Thermodynamic and Kinetic Effect of Biodegradable Polymers on Carbondioxide Hydrates. J. Ind. Eng. Chem. 2019, 79, 131–145. [Google Scholar] [CrossRef]
- Xu, S.; Fan, S.; Fang, S.; Lang, X.; Wang, Y.; Chen, J. Pectin as an Extraordinary Natural Kinetic Hydrate Inhibitor. Sci. Rep. 2016, 6, 23220. [Google Scholar] [CrossRef]
- Xu, P.; Lang, X.; Fan, S.; Wang, Y.; Chen, J. Molecular Dynamics Simulation of Methane Hydrate Growth in the Presence of the Natural Product Pectin. J. Phys. Chem. C 2016, 120, 5392–5397. [Google Scholar] [CrossRef]
- Drumond, B.; Drumond, B.; De Castro, J.; Fialho, B.; Vitorazi, L.; Ferraz, I. Pectin as natural gas hydrate inhibitor: Application of the avrami model. Braz. J. Petrol. Gas 2019, 13, 079–091. [Google Scholar] [CrossRef]
- Effendi, A.D.; Sia, C.W.; Jasamai, M.; Hashmani, M.A. Investigation on esterified pectin as natural hydrate inhibitor on methane hydrate formation. J. Petrol. Explor. Prod. Technol. 2022, 12, 3003–3019. [Google Scholar] [CrossRef]
- Wang, R.; Sun, H.; Shi, X.; Xu, X.; Zhang, L.; Zhang, Z. Fundamental Investigation of the Effects of Modified Starch, Carboxymethylcellulose Sodium, and Xanthan Gum on Hydrate Formation under Different Driving Forces. Energies 2019, 12, 2026. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.D.; Wu, H.; Englezos, P. Cationic Starches as Gas Hydrate Kinetic Inhibitors. Chem. Eng. Sci. 2007, 62, 6548–6555. [Google Scholar] [CrossRef]
- Talaghat, M.R. Experimental investigation of gas consumption for simple gas hydrate formation in a recirculation flow mini-loop apparatus in the presence of modified starch as a kinetic inhibitor. J. Nat. Gas Eng. 2013, 14, 42–48. [Google Scholar] [CrossRef]
- Talaghat, M.R. Enhancement of the performance of modified starch as a kinetic hydrate inhibitor in the presence of polyoxides for simple gas hydrate formation in a flow mini-loop apparatus. J. Nat. Gas Eng. 2014, 18, 7–12. [Google Scholar] [CrossRef]
- Fakharian, H.; Ganji, H.; Naderi Far, A.; Kameli, M. Potato Starch as Methane Hydrate Promoter. Fuel 2012, 94, 356–360. [Google Scholar] [CrossRef]
- Fakhreeva, A.V.; Voloshin, A.I.; Musin, F.F.; Telin, A.G.; Dokichev, V.A. Carboxymethylcellulose sodium salt—Effective “green” regent for management of calcium carbonate crystallization and natural gas hydrate formation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 525, 012050. [Google Scholar] [CrossRef] [Green Version]
- Ishmuratov, F.G.; Rakhimova, N.T.; Ishmiyarov, E.R.; Voloshin, A.I.; Gusakov, V.N.; Tomilov, Y.V.; Nifantyev, N.E.; Dokichev, V.A. New “green” polysaccharidal inhibitor of gas hydrate formation on the basis of carboxymethylcellulose sodium salt. Russ. J. Appl. Chem. 2018, 91, 653–656. [Google Scholar] [CrossRef]
- Dokichev, V.A.; Fakhreeva, A.V.; Voloshin, A.I.; Gusakov, V.N.; Ishmiyarov, E.R.; Grabovsky, S.A. Natrium salt of carboxymethyl cellulose as a basic reagent for creating a line of “green” oilfield reagents. Equip. Technol. Oil Gas Complex 2018, 5, 43–48. [Google Scholar] [CrossRef]
- Antonova, G.F.; Usov, A.I. Structure of arabinogalactan from siberian larch (Larix sibirica Ledeb) wood. Sov. J. Bioorg. Chem. 1984, 10, 1664–1669. [Google Scholar]
- Fu, W.; Wang, Z.; Chen, L.; Sun, B. Experimental Investigation of Methane Hydrate Formation in the Carboxmethylcellulose (CMC) Aqueous Solution. SPE J. 2020, 25, 1042–1056. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Yang, X.; Li, T. Synthesis of Chitosan Derivatives and Their Inhibition Effects on Methane Hydrates. Energies 2022, 15, 2675. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Shen, Y.; Yang, X.; Li, T.; Chen, G. The study on the relationship between the molecular structures of chitosan derivatives and their hydrate inhibition performance. J. Mol. Liq. 2022, 364, 120007. [Google Scholar] [CrossRef]

















| Type of Hydrate Inhibitors | Name of Chemical Reagents | Note | |
|---|---|---|---|
| High-Dosage Hydrate Inhibitors | Thermodynamic Hydrate Inhibitors | Glycols: MEG, TEG. Alcohols: MeOH, EtOH. Salts: NaCl, KCl. | Shift the hydrate-liquid-vapor equilibrium (HLVE) curve; applied in large quantities (10–50 wt%); generally anti-freezing solvents, i.e., methanol, glycols; ineffective in high-sub-cooling conditions. |
| Low-Dosage Hydrate Inhibitors | Green Hydrate Inhibitors | 1. Polysaccharides: chitosan–starch, cellulose ethers. 2. Anti-freeze proteins. | Shift the HLVE curve and retard hydrate formation; applied only at 0.5–2 wt%; generally water-soluble polymers. |
| Kinetic Hydrate Inhibitors | Polymers: seven-ring polyvinylcaprolactam, polyvinylpyrrolidone. Ionic liquids. | Delay or retard hydrate formation; applied only at 0.5–2 wt%; generally water-soluble polymers, i.e., PVP, PVCap; ineffective in high-sub-cooling conditions. | |
| Anti-Agglomerates | Sorbitan: Span20, Span80, Tween. | Do not allow particles to form hydrate plugs; applied only at 0.5–1 wt%; generally surfactants, i.e., Tween and Span series; ineffective in high-water-cut conditions. | |
| Concentration of Polysaccharide, % | Gas Hydrate Formation Start Pressure, Bar | Effective Rate Constant, r × 103, c−1 | Value of Reduction in the Rate of Gas Hydrate Formation, kMeOH/king | Effectiveness of Polysaccharide Inhibition α * = CMeOH/Cing |
|---|---|---|---|---|
| Na-CMC | ||||
| 0 | 143 | 4.11 | 1 | 1 |
| 0.005 | 168 | 3.57 | 1.15 | 214 |
| 0.0065 | 175 | 0.91 | 4.52 | 277 |
| 0.008 | 185 | 0.13 | 31.6 | 248 |
| Arabinogalactan | ||||
| 0 | 143 | 4.15 | 1 | 1 |
| 0.005 | 155 | 2.11 | 1.25 | 170 |
| 0.0065 | 167 | 0.812 | 5.11 | 231 |
| 0.008 | 184 | 0.193 | 35.5 | 263 |
| Dextran | ||||
| 0 | 143 | 4.39 | 1 | 1 |
| 0.005 | 169 | 2.47 | 1.18 | 290 |
| 0.0065 | 176 | 0.633 | 5.64 | 255 |
| 0.008 | 183 | 0.097 | 45.2 | 270 |
| Na-CMC | Dosage, % | Temperature of Gas Hydrate Formation, °C | Hydrate Formation Pressure, Bar | Effectiveness, α |
|---|---|---|---|---|
| Na-CMC-90 | 0 | 19 | 137 | 1 |
| 0.005 | 10.6 | 133 | 180 | |
| 0.010 | 5.0 | 131.5 | 200 | |
| 0.050 | 2.0 | 125 | 400 | |
| Na-CMC-250 | 0.005 | 16.6 | 133 | 1 |
| 0.010 | 13.7 | 137 | 40 | |
| 0.050 | −2.0 | 131 | 500 | |
| Na-CMC-700 | 0.005 | 19 | 138 | - |
| 0.010 | 19 | 136 | - | |
| 0.050 | 19 | 136 | - |
| Additives | PE (MPa) | Average Induction Time (h) | Growth Time (h) |
|---|---|---|---|
| Water | 4.14 | 2.80 | 9.9 |
| CS | 2.98 | 20.38 | 15.38 |
| HTCC | 2.96 | 5.90 | 16.53 |
| CMCS | 2.98 | 2.07 | 23.67 |
| HTCMCh | 3.03 | 7.70 | 19.12 |
| HBCC | 3.47 | 3.42 | 11.17 |
| H2ECC | 2.96 | 3.97 | 21.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakhreeva, A.V.; Nosov, V.V.; Voloshin, A.I.; Dokichev, V.A. Polysaccharides Are Effective Inhibitors of Natural Gas Hydrate Formation. Polymers 2023, 15, 1789. https://doi.org/10.3390/polym15071789
Fakhreeva AV, Nosov VV, Voloshin AI, Dokichev VA. Polysaccharides Are Effective Inhibitors of Natural Gas Hydrate Formation. Polymers. 2023; 15(7):1789. https://doi.org/10.3390/polym15071789
Chicago/Turabian StyleFakhreeva, Alsu Venerovna, Vasily Viktorovich Nosov, Alexander Iosifovich Voloshin, and Vladimir Anatolyevich Dokichev. 2023. "Polysaccharides Are Effective Inhibitors of Natural Gas Hydrate Formation" Polymers 15, no. 7: 1789. https://doi.org/10.3390/polym15071789
APA StyleFakhreeva, A. V., Nosov, V. V., Voloshin, A. I., & Dokichev, V. A. (2023). Polysaccharides Are Effective Inhibitors of Natural Gas Hydrate Formation. Polymers, 15(7), 1789. https://doi.org/10.3390/polym15071789