Stability in Aqueous Solution of a New Spray-Dried Hydrocolloid of High Andean Algae Nostoc sphaericum
Abstract
:1. Introduction
2. Materials and Methods
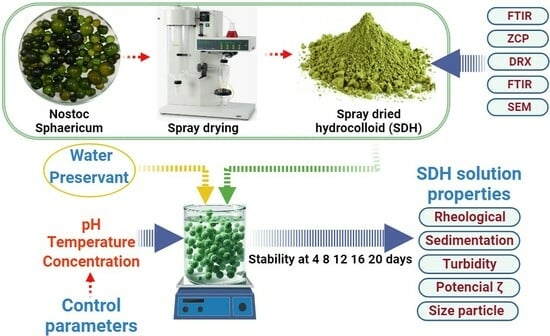
2.1. Raw Material
2.2. Preparation of Hydrocolloid Suspensions
2.3. SDH Characterization
2.4. Analysis of Rheological Behavior
2.5. Determination of Temperature Dependence
2.6. SDH Suspension Stability Evaluation
- If CI* −40 to −20, colors range from blue-violet to deep green.
- If CI* −20 to −2, the colors range from deep green to yellowish green.
- If CI* −2 to +2, represents greenish yellow.
- If CI* +2 to +20, colors range from pale yellow to deep orange.
- If CI* +20 to +40, colors range from deep orange to deep red.
2.7. Statistical Analysis
3. Results and Discussion
3.1. Zero Charge Point (ZCP)
3.2. Diffractometric Analysis and Degree of Crystallinity
3.3. FTIR Analysis
3.4. SEM Analysis
3.5. Rheological Analysis
3.6. Activation Energy of Solutions with SDH
3.7. SDH Suspension Stability
3.8. ζ Potential and Particle Size of SDH Suspension
3.9. PCA for the Treatments and Properties of the SDH Solution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Culetu, A.; Duta, D.E.; Papageorgiou, M.; Varzakas, T. The role of hydrocolloids in gluten-free bread and pasta; rheology, characteristics, staling and glycemic index. Foods 2021, 10, 3121. [Google Scholar] [CrossRef] [PubMed]
- Aftab, K.; Hameed, S.; Umbreen, H.; Ali, S.; Rizwan, M.; Alkahtani, S.; Abdel-Daim, M.M. Physicochemical and Functional Potential of Hydrocolloids Extracted from Some Solanaceae Plants. J. Chem. 2020, 2020, 3563945. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Lucas, B.F.; Alvarenga, A.G.P.; Moreira, J.B.; de Morais, M.G. Microalgae polysaccharides: An overview of production, characterization, and potential applications. Polysaccharides 2021, 2, 759–772. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Wang, W.; Li, Y. Advanced properties of gluten-free cookies, cakes, and crackers: A review. Trends Food Sci. Technol. 2020, 103, 200–213. [Google Scholar] [CrossRef]
- Gomes Gradíssimo, D.; Pereira Xavier, L.; Valadares Santos, A. Cyanobacterial polyhydroxyalkanoates: A sustainable alternative in circular economy. Molecules 2020, 25, 4331. [Google Scholar] [CrossRef]
- Jensen, S.; Petersen, B.O.; Omarsdottir, S.; Paulsen, B.S.; Duus, J.Ø.; Olafsdottir, E.S. Structural characterisation of a complex heteroglycan from the cyanobacterium Nostoc commune. Carbohydr. Polym. 2013, 91, 370–376. [Google Scholar] [CrossRef]
- Rodriguez, S.; Torres, F.G.; López, D. Preparation and characterization of polysaccharide films from the cyanobacteria Nostoc commune. Polym. Renew. Resour. 2017, 8, 133–150. [Google Scholar] [CrossRef]
- Fernández, L.A.G.; Ramos, V.C.; Polo, M.S.; Castillo, N.A.M. Fundamentals in applications of algae biomass: A review. J. Environ. Manag. 2023, 338, 117830. [Google Scholar] [CrossRef]
- Pérez-Lloréns, J.L. Microalgae: From staple foodstuff to avant-garde cuisine. Int. J. Gastron. Food Sci. 2020, 21, 100221. [Google Scholar] [CrossRef]
- Khalil, H.P.S.; Lai, T.K.; Tye, Y.Y.; Rizal, S.; Chong, E.W.N.; Yap, S.W.; Hamzah, A.A.; Fazita, M.R.; Paridah, M.T. A review of extractions of seaweed hydrocolloids: Properties and applications. Express Polym. Lett. 2018, 12, 296–317. [Google Scholar] [CrossRef]
- Ritzoulis, C.; Marini, E.; Aslanidou, A.; Georgiadis, N.; Karayannakidis, P.D.; Koukiotis, C.; Filotheou, A.; Lousinian, S.; Tzimpilis, E. Hydrocolloids from quince seed: Extraction, characterization, and study of their emulsifying/stabilizing capacity. Food Hydrocoll. 2014, 42, 178–186. [Google Scholar] [CrossRef]
- Corpus-Gomez, A.; Alcantara-Callata, M.; Celis-Teodoro, H.; Echevarria-Alarcón, B.; Paredes-Julca, J.; Paucar-Menacho, L.M. Cushuro (Nostoc sphaericum): Habitat, physicochemical characteristics, nutritional composition, forms of consumption and medicinal properties. Agroind. Sci. 2021, 11, 231–238. [Google Scholar] [CrossRef]
- Ponce, E. Nostoc: A different food and their presence in the precordillera of Arica. Idesia 2014, 32, 115–118. [Google Scholar] [CrossRef]
- Choque-Quispe, D.; Mojo-Quisani, A.; Ligarda-Samanez, C.A.; Calla-Florez, M.; Ramos-Pacheco, B.S.; Zamalloa-Puma, L.M.; Peralta-Guevara, D.E.; Solano-Reynoso, A.M.; Choque-Quispe, Y.; Zamalloa-Puma, A. Preliminary characterization of a spray-dried hydrocolloid from a high Andean algae (Nostoc sphaericum). Foods 2022, 11, 1640. [Google Scholar] [CrossRef]
- Pirsa, S.; Hafezi, K. Hydrocolloids: Structure, preparation method, and application in food industry. Food Chem. 2023, 399, 133967. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.S.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Rodriguez, S.; Gonzales, K.N.; Romero, E.G.; Troncoso, O.P.; Torres, F.G. Unusual reversible elastomeric gels from Nostoc commune. Int. J. Biol. Macromol. 2017, 97, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Nobes, D.S.; Vehring, R. Particle surface roughness improves colloidal stability of pressurized pharmaceutical suspensions. Pharm. Res. 2019, 36, 43. [Google Scholar] [CrossRef]
- Nep, E.I.; Conway, B.R. Evaluation of grewia polysaccharide gum as a suspending agent. Int. J. Pharm. Pharm. Sci. 2011, 3, 168–173. [Google Scholar]
- Koko, M.Y.F.; Hassanin, H.A.M.; Qi, B.; Han, L.; Lu, K.; Rokayya, S.; Harimana, Y.; Zhang, S.; Li, Y. Hydrocolloids as promising additives for food formulation consolidation: A short review. Food Rev. Int. 2023, 39, 1433–1439. [Google Scholar] [CrossRef]
- Zhang, Z. A new method for estimating zeta potential of carboxylic acids’ functionalised particles. Mol. Phys. 2023, e2260014. [Google Scholar] [CrossRef]
- Averkin, D.V.; Stakheev, A.A.; Vishnevetskii, D.V.; Pakhomov, P.M. Characterization of particles of the dispersed system based on low-concentrated aqueous solutions of L-cysteine and silver acetate. Proc. J. Phys. Conf. Ser. 2022, 2192, 012030. [Google Scholar] [CrossRef]
- Torres-Maza, A.; Yupanqui-Bacilio, C.; Castro, V.; Aguirre, E.; Villanueva, E.; Rodríguez, G. Comparison of the hydrocolloids Nostoc commune and Nostoc sphaericum: Drying, spectroscopy, rheology and application in nectar. Sci. Agropecu. 2020, 11, 583–589. [Google Scholar] [CrossRef]
- Ligarda-Samanez, C.A.; Choque-Quispe, D.; Palomino-Rincón, H.; Ramos-Pacheco, B.S.; Moscoso-Moscoso, E.; Huamán-Carrión, M.L.; Peralta-Guevara, D.E.; Obregón-Yupanqui, M.E.; Aroni-Huamán, J.; Bravo-Franco, E.Y.; et al. Modified Polymeric Biosorbents from Rumex acetosella for the Removal of Heavy Metals in Wastewater. Polymers 2022, 14, 2191. [Google Scholar] [CrossRef]
- Dome, K.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Changes in the crystallinity degree of starch having different types of crystal structure after mechanical pretreatment. Polymers 2020, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Emiola, I.; Adem, R. Comparison of Minimization Methods for Rosenbrock Functions. In Proceedings of the 2021 29th Mediterranean Conference on Control and Automation (MED), Puglia, Italy, 22–25 June 2021; pp. 837–842. [Google Scholar]
- Novati, P. Some secant approximations for Rosenbrock W-methods. Appl. Numer. Math. 2008, 58, 195–211. [Google Scholar] [CrossRef]
- He, H.; Lee, J.; Jiang, Z.; He, Q.; Dinic, J.; Chen, W.; Narayanan, S.; Lin, X.-M. Kinetics of Shear-Induced Structural Ordering in Dense Colloids. J. Phys. Chem. B 2023, 127, 7408–7415. [Google Scholar] [CrossRef]
- Martínez-Padilla, L.P. Rheology of liquid foods under shear flow conditions: Recently used models. J. Texture Stud. 2024, 55, e12802. [Google Scholar] [CrossRef]
- Choque-Quispe, D.; Froehner, S.; Ligarda-Samanez, C.A.; Ramos-Pacheco, B.S.; Palomino-Rincón, H.; Choque-Quispe, Y.; Solano-Reynoso, A.M.; Taipe-Pardo, F.; Zamalloa-Puma, L.M.; Calla-Florez, M. Preparation and chemical and physical characteristics of an edible film based on native potato starch and nopal mucilage. Polymers 2021, 13, 3719. [Google Scholar] [CrossRef]
- Hadimani, L.; Mittal, N. Development of a computer vision system to estimate the colour indices of Kinnow mandarins. J. Food Sci. Technol. 2019, 56, 2305–2311. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.K.; Alazhary, A.M.; Al-Zaqri, N.; Alsalme, A.; Alharthi, F.A.; Hamdy, M.S. Cost-effective adsorbent from arabinogalactan and pectin of cactus pear peels: Kinetics and thermodynamics studies. Int. J. Biol. Macromol. 2020, 150, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Salminen, H.; Weiss, J. Effect of pectin type on association and pH stability of whey protein—Pectin complexes. Food Biophys. 2014, 9, 29–38. [Google Scholar] [CrossRef]
- Ahmad, R.; Mirza, A. Synthesis of Guar gum/bentonite a novel bionanocomposite: Isotherms, kinetics and thermodynamic studies for the removal of Pb (II) and crystal violet dye. J. Mol. Liq. 2018, 249, 805–814. [Google Scholar] [CrossRef]
- Arumugham, T.; Krishnamoorthy, R.; AlYammahi, J.; Hasan, S.W.; Banat, F. Spray dried date fruit extract with a maltodextrin/gum arabic binary blend carrier agent system: Process optimization and product quality. Int. J. Biol. Macromol. 2023, 238, 124340. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, W. Characterisation of spray dried soy sauce powders made by adding crystalline carbohydrates to drying carrier. Food Chem. 2015, 168, 417–422. [Google Scholar] [CrossRef]
- Nazir, S.; Wani, I.A. Fractionation and characterization of mucilage from Basil (Ocimum basilicum L.) seed. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100429. [Google Scholar] [CrossRef]
- Karrar, E.; Mahdi, A.A.; Sheth, S.; Ahmed, I.A.M.; Manzoor, M.F.; Wei, W.; Wang, X. Effect of maltodextrin combination with gum arabic and whey protein isolate on the microencapsulation of gurum seed oil using a spray-drying method. Int. J. Biol. Macromol. 2021, 171, 208–216. [Google Scholar] [CrossRef]
- Chuenkaek, T.; Nakajima, K.; Kobayashi, T. Water-soluble pectin films prepared with extracts from Citrus maxima wastes under acidic conditions and their moisturizing characteristics. Biomass Convers. Biorefinery 2023, 1–14. [Google Scholar] [CrossRef]
- Supreetha, R.; Bindya, S.; Deepika, P.; Vinusha, H.M.; Hema, B.P. Characterization and biological activities of synthesized citrus pectin-MgO nanocomposite. Results Chem. 2021, 3, 100156. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Ciftci, O.N. Fabrication of bioaerogels from camelina seed mucilage for food applications. Food Hydrocoll. 2020, 102, 105597. [Google Scholar] [CrossRef]
- Alpizar-Reyes, E.; Carrillo-Navas, H.; Gallardo-Rivera, R.; Varela-Guerrero, V.; Alvarez-Ramirez, J.; Pérez-Alonso, C. Functional properties and physicochemical characteristics of tamarind (Tamarindus indica L.) seed mucilage powder as a novel hydrocolloid. J. Food Eng. 2017, 209, 68–75. [Google Scholar] [CrossRef]
- González-Martínez, D.A.; Carrillo-Navas, H.; Barrera-Díaz, C.E.; Martínez-Vargas, S.L.; Alvarez-Ramírez, J.; Pérez-Alonso, C. Characterization of a novel complex coacervate based on whey protein isolate-tamarind seed mucilage. Food Hydrocoll. 2017, 72, 115–126. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Li, C.; Xu, Y.; Luo, Y.; Liang, D.; Huang, C. Comprehensive review of polysaccharide-based materials in edible packaging: A sustainable approach. Foods 2021, 10, 1845. [Google Scholar] [CrossRef] [PubMed]
- Chakravartula, S.S.N.; Soccio, M.; Lotti, N.; Balestra, F.; Dalla Rosa, M.; Siracusa, V. Characterization of composite edible films based on pectin/alginate/whey protein concentrate. Materials 2019, 12, 2454. [Google Scholar] [CrossRef] [PubMed]
- Hamrun, N.; Talib, B.; Ruslin, M.; Pangeran, H.; Hatta, M.; Marlina, E.; Yusuf, A.S.H.; Saito, T.; Ou, K.-L. A promising potential of brown algae Sargassum polycystum as irreversible hydrocolloid impression material. Mar. Drugs 2022, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- Sebeia, N.; Jabli, M.; Ghith, A.; Elghoul, Y.; Alminderej, F.M. Production of cellulose from Aegagropila Linnaei macro-algae: Chemical modification, characterization and application for the bio-sorptionof cationic and anionic dyes from water. Int. J. Biol. Macromol. 2019, 135, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Duan, H.; Wang, C.; Huang, X. Characteristics of two calcium pectinates prepared from citrus pectin using either calcium chloride or calcium hydroxide. J. Agric. Food Chem. 2014, 62, 6354–6361. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Wang, B.; Adhikari, R.; Adhikari, B. Preparation and characterization of chia seed protein isolate–chia seed gum complex coacervates. Food Hydrocoll. 2016, 52, 554–563. [Google Scholar] [CrossRef]
- Chuquilín-Goicochea, R.; Ccente-Lulo, F.; Arteaga-Llacza, P.; Huayta, F.; Tinoco, H.A.O. Chemical and rheological characterization of Senna birostris hydrocolloid. Manglar 2021, 18, 11–115. [Google Scholar] [CrossRef]
- Temsiripong, T.; Pongsawatmanit, R.; Ikeda, S.; Nishinari, K. Influence of xyloglucan on gelatinization and retrogradation of tapioca starch. Food Hydrocoll. 2005, 19, 1054–1063. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Q.; Zhao, J.; Lan, X.; Yao, K.; Jia, D. Physicochemical and rheological properties of crude polysaccharides extracted from Tremella fuciformis with different methods. CyTA-J. Food 2021, 19, 247–256. [Google Scholar] [CrossRef]
- Okechukwu, P.E.; Rao, M.A. Role of granule size and size distribution in the viscosity of cowpea starch dispersions heated in excess water. J. Texture Stud. 1996, 27, 159–173. [Google Scholar] [CrossRef]
- Hernández-Morales, M.d.l.Á.; Maldonado-Astudillo, Y.I.; Jiménez-Hernández, J.; Salazar, R.; Ramírez-Sucre, M.O.; Ibarz, A.; Utrilla-Coello, R.G.; Ortuño-Pineda, C. Physicochemical and rheological properties of gum seed and pulp from Hymenaea courbaril L. CyTA-J. Food 2018, 16, 986–994. [Google Scholar] [CrossRef]
- Yekta, M.; Ansari, S. Jujube mucilage as a potential stabilizer in stirred yogurt: Improvements in the physiochemical, rheological, and sensorial properties. Food Sci. Nutr. 2019, 7, 3709–3721. [Google Scholar] [CrossRef] [PubMed]
- León-Martínez, F.M.; Cano-Barrita, P.F.J.; Lagunez-Rivera, L.; Medina-Torres, L. Study of nopal mucilage and marine brown algae extract as viscosity-enhancing admixtures for cement based materials. Constr. Build. Mater. 2014, 53, 190–202. [Google Scholar] [CrossRef]
- Rivera-Corona, J.L.; Rodríguez-González, F.; Rendón-Villalobos, R.; García-Hernández, E.; Solorza-Feria, J. Thermal, structural and rheological properties of sorghum starch with cactus mucilage addition. LWT-Food Sci. Technol. 2014, 59, 806–812. [Google Scholar] [CrossRef]
- Zhou, M.; Robards, K.; Glennie-Holmes, M.; Helliwell, S. Structure and pasting properties of oat starch. Cereal Chem. 1998, 75, 273–281. [Google Scholar] [CrossRef]
- Mohamed, I.O. Effects of processing and additives on starch physicochemical and digestibility properties. Carbohydr. Polym. Technol. Appl. 2021, 2, 100039. [Google Scholar] [CrossRef]
- Rady, A.M.; Soliman, S.N.; El-Wersh, A. Effect of mechanical treatments on creep behavior of potato tubers. Eng. Agric. Environ. Food 2017, 10, 282–291. [Google Scholar] [CrossRef]
- Alpizar-Reyes, E.; Román-Guerrero, A.; Gallardo-Rivera, R.; Varela-Guerrero, V.; Cruz-Olivares, J.; Pérez-Alonso, C. Rheological properties of tamarind (Tamarindus indica L.) seed mucilage obtained by spray-drying as a novel source of hydrocolloid. Int. J. Biol. Macromol. 2018, 107, 817–824. [Google Scholar] [CrossRef]
- Chin, N.L.; Chan, S.M.; Yusof, Y.A.; Chuah, T.G.; Talib, R.A. Modelling of rheological behaviour of pummelo juice concentrates using master-curve. J. Food Eng. 2009, 93, 134–140. [Google Scholar] [CrossRef]
- García-Cruz, E.E.; Rodriguez-Ramirez, J.; Lagunas, L.L.M.; Medina-Torres, L. Rheological and physical properties of spray-dried mucilage obtained from Hylocereus undatus cladodes. Carbohydr. Polym. 2013, 91, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, G.; Liu, T.; Chen, Y.; Gong, H. The comparison of rheological properties of aqueous welan gum and xanthan gum solutions. Carbohydr. Polym. 2013, 92, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Dak, M.; Verma, R.C.; Jaaffrey, S.N.A. Effect of temperature and concentration on rheological properties of “Kesar” mango juice. J. Food Eng. 2007, 80, 1011–1015. [Google Scholar] [CrossRef]
- Quispe-Chambilla, L.; Pumacahua-Ramos, A.; Choque-Quispe, D.; Curro-Pérez, F.; Carrión-Sánchez, H.M.; Peralta-Guevara, D.E.; Masco-Arriola, M.L.; Palomino-Rincón, H.; Ligarda-Samanez, C.A. Rheological and functional properties of dark chocolate with partial substitution of peanuts and sacha inchi. Foods 2022, 11, 1142. [Google Scholar] [CrossRef]
- Kassem, I.A.A.; Ashaolu, T.J.; Kamel, R.; Elkasabgy, N.A.; Afifi, S.M.; Farag, M.A. Mucilage as a functional food hydrocolloid: Ongoing and potential applications in prebiotics and nutraceuticals. Food Funct. 2021, 12, 4738–4748. [Google Scholar] [CrossRef] [PubMed]
- Medina-Torres, L.; García-Cruz, E.E.; Calderas, F.; Laredo, R.F.G.; Sánchez-Olivares, G.; Gallegos-Infante, J.A.; Rocha-Guzmán, N.E.; Rodriguez-Ramirez, J. Microencapsulation by spray drying of gallic acid with nopal mucilage (Opuntia ficus indica). LWT-Food Sci. Technol. 2013, 50, 642–650. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, M.; Yang, C.; Wang, Z. The Growth of Precipitates in A Solution with Cross Diffusion Between Solutes. Surf. Rev. Lett. 2022, 29, 2250148. [Google Scholar] [CrossRef]
- Alexander, K.S.; Biskup, M.; Chayes, L. Colligative properties of solutions: II. Vanishing concentrations. J. Stat. Phys. 2005, 119, 509–537. [Google Scholar] [CrossRef]
- Kosmulski, M.; Mączka, E. Zeta potential and particle size in dispersions of alumina in 50–50 w/w ethylene glycol-water mixture. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130168. [Google Scholar] [CrossRef]
- Ishaq, A.; Nadeem, M.; Ahmad, R.; Ahmed, Z.; Khalid, N. Recent advances in applications of marine hydrocolloids for improving bread quality. Food Hydrocoll. 2023, 148, 109424. [Google Scholar] [CrossRef]
- Chatterjee, A.; Lal, S.; Manivasagam, T.G.; Batabyal, S.K. Surface charge induced bioelectricity generation from freshwater macroalgae Pithophora. Bioresour. Technol. Rep. 2023, 22, 101379. [Google Scholar] [CrossRef]
- Niknam, R.; Mousavi, M.; Kiani, H. Intrinsic viscosity, steady and oscillatory shear rheology of a new source of galactomannan isolated from Gleditsia caspica (Persian honey locust) seeds in aqueous dispersions. Eur. Food Res. Technol. 2021, 247, 2579–2590. [Google Scholar] [CrossRef]
- Strieder, M.M.; Silva, E.K.; Mekala, S.; Meireles, M.A.A.; Saldaña, M.D.A. Barley-Based Non-dairy Alternative Milk: Stabilization Mechanism, Protein Solubility, Physicochemical Properties, and Kinetic Stability. Food Bioprocess Technol. 2023, 16, 2231–2246. [Google Scholar] [CrossRef]
- Marsiglia-Fuentes, R.; Quintana, S.E.; García Zapateiro, L.A. Novel Hydrocolloids Obtained from Mango (Mangifera indica) var. Hilaza: Chemical, Physicochemical, Techno-Functional, and Structural Characteristics. Gels 2022, 8, 354. [Google Scholar] [CrossRef]









| Treatment | Factor 1: pH | Factor 2: Concentration (ppm) | Factor 3: Temperature (°C) |
|---|---|---|---|
| T1 | 6.5 | 100 | 60 |
| T2 | 6.5 | 100 | 80 |
| T3 | 4.5 | 100 | 60 |
| T4 | 4.5 | 100 | 80 |
| T5 | 6.5 | 70 | 60 |
| T6 | 6.5 | 70 | 80 |
| T7 | 4.5 | 70 | 60 |
| T8 | 4.5 | 70 | 80 |
| T9 | 6.5 | 100 | 40 |
| T10 | 6.5 | 70 | 40 |
| T11 | 4.5 | 100 | 40 |
| T12 | 4.5 | 70 | 40 |
| Model | Equation | Parameters | |
|---|---|---|---|
| Power law | (3) | ||
| Herschel–Bulkley | (4) | ||
| Casson | (5) |
| Model | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | T11 | T12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Power Law | ||||||||||||
| k(Pa.sn × 10−4) | 2.5042 | 1.7931 | 1.1154 | 0.7935 | 1.3742 | 1.1315 | 0.9657 | 0.6444 | 3.6937 | 1.5130 | 3.4568 | 1.8615 |
| n | 1.3772 | 1.4125 | 1.5196 | 1.5741 | 1.4457 | 1.5068 | 1.5473 | 1.6229 | 1.2557 | 1.4010 | 1.2951 | 1.4350 |
| R2 | 0.9942 | 0.9898 | 0.9996 | 0.9999 | 0.9995 | 0.9997 | 0.9996 | 0.9999 | 0.9883 | 0.9998 | 0.9950 | 0.9986 |
| MSE | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0001 | 0.0001 |
| MAPE | 0.0892 | 0.1149 | 0.0703 | 0.0568 | 0.0495 | 0.0599 | 0.1522 | 0.0659 | 0.3849 | 0.0587 | 0.1107 | 0.0747 |
| Residuals | R | R | R | R | R | R | R | R | R | R | R | R |
| EM | QN | QN | QN | QN | QN | QN | QN | QN | QN | QN | QN | QN |
| Herschel-Bulkley | ||||||||||||
| (Pa × 10−4) | 53.4769 | 56.4827 | 44.5463 | 31.8798 | 0.0000 | 18.7968 | 55.7519 | 53.5837 | 0.0000 | 0.0000 | 0.0000 | 0.1239 |
| kH(Pa.sn × 10−4) | 1.2746 | 0.7209 | 0.9518 | 0.7023 | 1.3742 | 1.0556 | 0.7876 | 0.5272 | 3.6938 | 1.5131 | 3.4568 | 1.8579 |
| n | 1.5185 | 1.6041 | 1.5466 | 1.5949 | 1.4457 | 1.5187 | 1.5821 | 1.6573 | 1.2557 | 1.4010 | 1.2951 | 1.4354 |
| R2 | 0.9961 | 0.9929 | 0.9997 | 0.9999 | 0.9995 | 0.9997 | 0.9997 | 0.9999 | 0.9968 | 0.9997 | 0.9958 | 0.9988 |
| MSE | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0001 | 0.0001 |
| MAPE | 0.0579 | 0.0990 | 0.0320 | 0.0216 | 0.0495 | 0.0452 | 0.4167 | 0.0179 | 0.3850 | 0.0587 | 0.1107 | 0.0745 |
| Residuals | R | R | R | R | R | R | R | R | R | R | T | R |
| EM | QN | QN | QN | QN | QN | QN | QN | QN | QN | QN | QN | QN |
| Casson | ||||||||||||
| (Pa × 10−4) | 0.0109 | 0.0423 | 0.2735 | 0.4306 | 2.0557 | 0.3171 | 0.1104 | 0.0620 | 0.0158 | 0.3009 | 0.1378 | 0.0247 |
| k(Pa.sn × 10−4) | 11.834 | 10.060 | 17.718 | 17.189 | 14.458 | 17.119 | 18.020 | 18.452 | 10.530 | 13.066 | 16.298 | 19.236 |
| R2 | 0.9456 | 0.9426 | 0.9353 | 0.9270 | 0.9433 | 0.9385 | 0.9329 | 0.9211 | 0.9630 | 0.9545 | 0.9656 | 0.9507 |
| MSE | 0.0001 | 0.0001 | 0.0024 | 0.0026 | 0.0014 | 0.0021 | 0.0026 | 0.0032 | 0.0001 | 0.0008 | 0.0010 | 0.0020 |
| MAPE | 0.2130 | 0.2187 | 0.4176 | 0.5376 | 0.4910 | 0.4587 | 1.1148 | 0.5534 | 0.6942 | 0.4808 | 0.3843 | 0.4534 |
| Residuals | T | T | T | T | T | T | T | T | R | T | T | T |
| EM | SQN | SQN | QN | SQN | SQN | SQN | QN | SQN | SQN | SQN | SQN | SQN |
| Treatment | Ea (kJ/mol) | R2 | ||
|---|---|---|---|---|
| pH | ppm | T (°C) | ||
| 4.5 | 70 | 40 | 29.127 | 0.9708 |
| 4.5 | 70 | 60 | ||
| 4.5 | 70 | 80 | ||
| 4.5 | 100 | 40 | 37.043 | 0.9079 |
| 4.5 | 100 | 60 | ||
| 4.5 | 100 | 80 | ||
| 6.5 | 70 | 40 | 8.190 | 0.9144 |
| 6.5 | 70 | 60 | ||
| 6.5 | 70 | 80 | ||
| 6.5 | 100 | 40 | 37.740 | 0.981 |
| 6.5 | 100 | 60 | ||
| 6.5 | 100 | 80 | ||
| Treatment | Turbidity | Sedimentation | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | p-Value * | Day | p-Value * | |||||||||||||
| 0 | 1 | 4 | 8 | 12 | 16 | 20 | 0 | 1 | 4 | 8 | 12 | 16 | 20 | |||
| T1 | a | a | c | e | d | d | c | <0.05 | -- | b | a | c | c | d | a | <0.05 |
| T2 | b | ab | b | e | c | d | c | <0.05 | -- | b | b | c | d | d | b | <0.05 |
| T3 | c | ab | c | d | c | cd | c | <0.05 | -- | c | c | e | e | e | c | <0.05 |
| T4 | d | a | c | d | c | d | c | <0.05 | -- | d | d | d | e | e | d | <0.05 |
| T5 | e | ab | ab | cd | b | bc | b | <0.05 | -- | a | e | a | a | b | e | <0.05 |
| T6 | f | b | a | bc | a | a | a | <0.05 | -- | c,d | f | b | b | a | f | <0.05 |
| T7 | g | ab | a | a | a | ab | ab | <0.05 | -- | e | g | c | b | c | g | <0.05 |
| T8 | h | ab | ab | ab | a | ab | a | <0.05 | -- | c,d | h | c | b | d | h | <0.05 |
| Treatment | ζ Potential (mV) | Size Particle (nm) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | p-Value * | Day | p-Value * | |||||||||||
| 1 | 4 | 8 | 12 | 16 | 20 | 1 | 4 | 8 | 12 | 16 | 20 | |||
| T1 | a | a,b | c | a | b | b | <0.05 | a | a | c,d | c,d | c | b | <0.05 |
| T2 | a | a | b | a,b | b | b | <0.05 | d | b | b | a,b | c | b | <0.05 |
| T3 | a | a,b,c | d | c | a,b | b | <0.05 | d | a,b | a,b | a,b | a | a | <0.05 |
| T4 | a | a,b,c | c | c | a,b | b | <0.05 | a,b | b,c | c,d | c,d | b,c | b | <0.05 |
| T5 | a | d | a | a | a | b | <0.05 | c,d | a,b | a | a | a | a | <0.05 |
| T6 | a | c | a | a,b,c | a,b | b | <0.05 | c,d | d,e | c | c | c | b | <0.05 |
| T7 | a | a,b,c | b | b,c | a | a | <0.05 | b,c | c,d | c | b,c | a,b | a | <0.05 |
| T8 | a | b,c | c,d | c | a | a,b | <0.05 | c,d | e | d | d | c | b | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choque-Quispe, D.; Ligarda-Samanez, C.A.; Choque-Quispe, Y.; Froehner, S.; Solano-Reynoso, A.M.; Moscoso-Moscoso, E.; Carhuarupay-Molleda, Y.F.; Peréz-Salcedo, R. Stability in Aqueous Solution of a New Spray-Dried Hydrocolloid of High Andean Algae Nostoc sphaericum. Polymers 2024, 16, 537. https://doi.org/10.3390/polym16040537
Choque-Quispe D, Ligarda-Samanez CA, Choque-Quispe Y, Froehner S, Solano-Reynoso AM, Moscoso-Moscoso E, Carhuarupay-Molleda YF, Peréz-Salcedo R. Stability in Aqueous Solution of a New Spray-Dried Hydrocolloid of High Andean Algae Nostoc sphaericum. Polymers. 2024; 16(4):537. https://doi.org/10.3390/polym16040537
Chicago/Turabian StyleChoque-Quispe, David, Carlos A. Ligarda-Samanez, Yudith Choque-Quispe, Sandro Froehner, Aydeé M. Solano-Reynoso, Elibet Moscoso-Moscoso, Yakov Felipe Carhuarupay-Molleda, and Ronald Peréz-Salcedo. 2024. "Stability in Aqueous Solution of a New Spray-Dried Hydrocolloid of High Andean Algae Nostoc sphaericum" Polymers 16, no. 4: 537. https://doi.org/10.3390/polym16040537
APA StyleChoque-Quispe, D., Ligarda-Samanez, C. A., Choque-Quispe, Y., Froehner, S., Solano-Reynoso, A. M., Moscoso-Moscoso, E., Carhuarupay-Molleda, Y. F., & Peréz-Salcedo, R. (2024). Stability in Aqueous Solution of a New Spray-Dried Hydrocolloid of High Andean Algae Nostoc sphaericum. Polymers, 16(4), 537. https://doi.org/10.3390/polym16040537