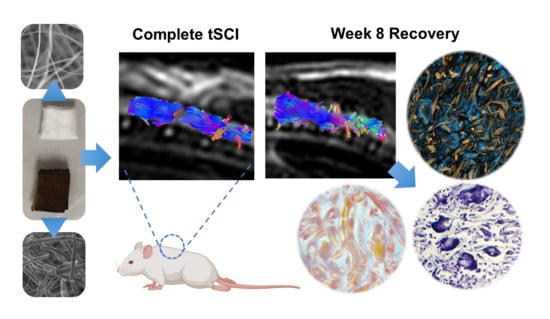
Improved Recovery of Complete Spinal Cord Transection by a Plasma-Modified Fibrillar Scaffold
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Scaffold Design
2.3. Animals
2.4. Study Design
2.5. Spinal Cord Transection
2.6. Magnetic Resonance Imaging (MRI) Acquisition
2.7. Locomotion Analysis
2.8. Histology, Immunofluorescence, and Confocal Microscopy
2.9. Statistical Analysis
3. Results
3.1. Scaffold Design and Rapid Tissue Response to Plasma-Modified Scaffolds
3.2. Implanted Animals Showed Improved Motor Recovery
3.3. Scaffolds Promoted Tissue Growth at the Injury Site
3.4. Recovery of Structural Damage by the Fibrillar Scaffolds
3.5. The Recovery of Anisotropy Baseline Values Suggests Neural Pathway Reorganization across the Scaffolds
3.6. The Fibrillar Scaffolds Promoted Metabolic Changes in Response to Injury
3.7. The Plasma Modification of the Fibers Mitigated Tissue Degeneration
3.8. Evidence of Neuronal Marker MAP2 over the Fibers Demonstrated Fibrillar Scaffolds Are Potential Substrates for Nerve Repair
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic Spinal Cord Injury. Nat. Rev. Dis. Prim. 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.; Das, J.; Emmady, P. Spinal Cord Injuries. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560721/ (accessed on 7 March 2024).
- Flack, J.; Sharma, K.; Xie, J. Delving into the Recent Advancements of Spinal Cord Injury Treatment: A Review of Recent Progress. Neural Regen. Res. 2022, 17, 283–291. [Google Scholar] [CrossRef]
- Grijalva-Otero, I.; Doncel-Pérez, E. Traumatic Human Spinal Cord Injury: Are Single Treatments Enough to Solve the Problem? Arch. Med. Res. 2024, 55, 102935. [Google Scholar] [CrossRef]
- Zustiak, S.P.; Sheth, S.; Imaninezhad, M. Pharmacological Therapies and Factors Delivery for Spinal Cord Injury Regeneration. In Spinal Cord Injury (SCI) Repair Strategies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 223–248. ISBN 9780081028070. [Google Scholar]
- Hu, X.; Xu, W.; Ren, Y.; Wang, Z.; He, X.; Huang, R.; Ma, B.; Zhao, J.; Zhu, R.; Cheng, L. Spinal Cord Injury: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 245. [Google Scholar] [CrossRef]
- Oña, A.; Athanasios, K.; Tederko, P.; Escorpizo, R.; Arora, M.; Sturm, C.; Yang, S.; Barzallo, D.P. Unmet Healthcare Needs and Health Inequalities in People with Spinal Cord Injury: A Direct Regression Inequality Decomposition. Int. J. Equity Health 2023, 22, 56. [Google Scholar] [CrossRef]
- Forte, G.; Giuffrida, V.; Scuderi, A.; Pazzaglia, M. Future Treatment of Neuropathic Pain in Spinal Cord Injury: The Challenges of Nanomedicine, Supplements or Opportunities? Biomedicines 2022, 10, 1373. [Google Scholar] [CrossRef] [PubMed]
- Guízar-Sahagún, G.; Grijalva, I.; Franco-Bourland, R.E.; Madrazo, I. Aging with Spinal Cord Injury: A Narrative Review of Consequences and Challenges. Ageing Res. Rev. 2023, 90, 102020. [Google Scholar] [CrossRef]
- Patek, M.; Stewart, M. Spinal Cord Injury. Anaesth. Intensive Care Med. 2023, 24, 406–411. [Google Scholar] [CrossRef]
- Chandra, J.; Sheerin, F.; Lopez De Heredia, L.; Meagher, T.; King, D.; Belci, M.; Hughes, R.J. MRI in Acute and Subacute Post-Traumatic Spinal Cord Injury: Pictorial Review. Spinal Cord 2012, 50, 2–7. [Google Scholar] [CrossRef]
- Kim, K.D.; Lee, K.S.; Coric, D.; Harrop, J.S.; Theodore, N.; Toselli, R.M. Acute Implantation of a Bioresorbable Polymer Scaffold in Patients with Complete Thoracic Spinal Cord Injury: 24-Month Follow-up from the INSPIRE Study. Neurosurgery 2022, 90, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Morales-Guadarrama, A.; Salgado-Ceballos, H.; Grijalva, I.; Morales-Corona, J.; Hernández-Godínez, B.; Ibáñez-Contreras, A.; Ríos, C.; Diaz-Ruiz, A.; Cruz, G.J.; Olayo, M.G.; et al. Evolution of Spinal Cord Transection of Rhesus Monkey Implanted with Polymer Synthesized by Plasma Evaluated by Diffusion Tensor Imaging. Polymers 2022, 14, 962. [Google Scholar] [CrossRef] [PubMed]
- Theodore, N.; Hlubek, R.; Danielson, J.; Neff, K.; Vaickus, L.; Ulich, T.R.; Ropper, A.E. First Human Implantation of a Bioresorbable Polymer Scaffold for Acute Traumatic Spinal Cord Injury: A Clinical Pilot Study for Safety and Feasibility. Neurosurgery 2016, 79, E305–E312. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.D.; Moore, S.W.; Aimetti, A.A.; Kutikov, A.B.; Santamaria, A.J.; Hofstetter, C.P.; Ropper, A.E.; Theodore, N.; Ulich, T.R.; Layer, R.T. Internal Decompression of the Acutely Contused Spinal Cord: Differential Effects of Irrigation Only versus Biodegradable Scaffold Implantation. Biomaterials 2018, 185, 284–300. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Lim, J.; Mekary, R.A.; Rattani, A.; Dewan, M.C.; Sharif, S.Y.; Osorio-Fonseca, E.; Park, K.B. Traumatic Spinal Injury: Global Epidemiology and Worldwide Volume. World Neurosurg. 2018, 113, e345–e363. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Xiao, Z.; Zhao, Y.; Wang, B.; Li, X.; Li, J.; Dai, J. Collagen Scaffold Combined with Human Umbilical Cord-Derived Mesenchymal Stem Cells Promote Functional Recovery after Scar Resection in Rats with Chronic Spinal Cord Injury. J. Tissue Eng. Regen. Med. 2018, 12, e1154–e1163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, F.; Xiao, Z.; Han, G.; Wang, N.; Yin, N.; Chen, B.; Jiang, X.; Yun, C.; Han, W.; et al. Clinical Study of NeuroRegen Scaffold Combined with Human Mesenchymal Stem Cells for the Repair of Chronic Complete Spinal Cord Injury. Cell Transplant. 2017, 26, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Fan, C.; You, Z.; Xiao, Z.; Zhao, Y.; Dai, J. Advances in Biomaterial-Based Spinal Cord Injury Repair. Adv. Funct. Mater. 2022, 32, 2110628. [Google Scholar] [CrossRef]
- Gelain, F.; Panseri, S.; Antonini, S.; Cunha, C.; Donega, M.; Lowery, J.; Taraballi, F.; Cerri, G.; Montagna, M.; Baldissera, F.; et al. Transplantation of Nanostructured Composite Scaffolds Results in the Regeneration of Chronically Injured Spinal Cords. ACS Nano 2011, 5, 227–236. [Google Scholar] [CrossRef]
- Mutepfa, A.R.; Hardy, J.G.; Adams, C.F. Electroactive Scaffolds to Improve Neural Stem Cell Therapy for Spinal Cord Injury. Front. Med. Technol. 2022, 4, 693438. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, Y.; Wu, H. Polymeric Fibers as Scaffolds for Spinal Cord Injury: A Systematic Review. Front. Bioeng. Biotechnol. 2022, 9, 807533. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Imajo, Y.; Funaba, M.; Ikeda, H.; Nishida, N.; Sakai, T. Current Concepts of Biomaterial Scaffolds and Regenerative Therapy for Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 2528. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.; Levenberg, S. The Role of Biomaterials in Peripheral Nerve and Spinal Cord Injury: A Review. Int. J. Mol. Sci. 2022, 23, 1244. [Google Scholar] [CrossRef] [PubMed]
- Faccendini, A.; Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Nanofiber Scaffolds as Drug Delivery Systems to Bridge Spinal Cord Injury. Pharmaceuticals 2017, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.D.; Lee, K.S.; Coric, D.; Chang, J.J.; Harrop, J.S.; Theodore, N.; Toselli, R.M. A Study of Probable Benefit of a Bioresorbable Polymer Scaffold for Safety and Neurological Recovery in Patients with Complete Thoracic Spinal Cord Injury: 6-Month Results from the INSPIRE Study. J. Neurosurg. Spine 2021, 34, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Tang, F.; Tang, J.; Yang, H.; Zhao, Y.; Chen, B.; Han, S.; Wang, N.; Li, X.; Cheng, S.; et al. One-Year Clinical Study of NeuroRegen Scaffold Implantation Following Scar Resection in Complete Chronic Spinal Cord Injury Patients. Sci. China Life Sci. 2016, 59, 647–655. [Google Scholar] [CrossRef]
- Sánchez-Torres, S.; Díaz-Ruíz, A.; Ríos, C.; Olayo, M.G.; Cruz, G.J.; Olayo, R.; Morales, J.; Mondragón-Lozano, R.; Fabela-Sánchez, O.; Orozco-Barrios, C.; et al. Recovery of Motor Function after Traumatic Spinal Cord Injury by Using Plasma-Synthesized Polypyrrole/Iodine Application in Combination with a Mixed Rehabilitation Scheme. J. Mater. Sci. Mater. Med. 2020, 31, 58. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Mejía, L.; Morales, J.; Cruz, G.J.; Olayo, M.-G.; Olayo, R.; Díaz-Ruíz, A.; Ríos, C.; Mondragón-Lozano, R.; Sánchez-Torres, S.; Morales-Guadarrama, A.; et al. Functional Recovery in Spinal Cord Injured Rats Using Polypyrrole/Iodine Implants and Treadmill Training. J. Mater. Sci. Mater. Med. 2015, 26, 209. [Google Scholar] [CrossRef]
- Mondragon-Lozano, R.; Ríos, C.; Roldan-Valadez, E.; Cruz, G.J.; Olayo, M.G.; Olayo, R.; Salgado-Ceballos, H.; Morales, J.; Mendez-Armenta, M.; Alvarez-Mejia, L.; et al. Delayed Injection of Polypyrrole Doped with Iodine Particle Suspension after Spinal Cord Injury in Rats Improves Functional Recovery and Decreased Tissue Damage Evaluated by 3.0 Tesla in Vivo Magnetic Resonance Imaging. Spine J. 2017, 17, 562–573. [Google Scholar] [CrossRef]
- Fabela-Sánchez, O.; Salgado–Ceballos, H.; Medina-Torres, L.; Álvarez-Mejía, L.; Sánchez-Torres, S.; Mondragón-Lozano, R.; Morales-Guadarrama, A.; Díaz-Ruiz, A.; Olayo, M.-G.; Cruz, G.J.; et al. Effect of the Combined Treatment of Albumin with Plasma Synthesised Pyrrole Polymers on Motor Recovery after Traumatic Spinal Cord Injury in Rats. J. Mater. Sci. Mater. Med. 2018, 29, 13. [Google Scholar] [CrossRef]
- Olayo, R.; Ríos, C.; Salgado-Ceballos, H.; Cruz, G.J.; Morales, J.; Olayo, M.G.; Alcaraz-Zubeldia, M.; Alvarez, A.L.; Mondragon, R.; Morales, A.; et al. Tissue Spinal Cord Response in Rats after Implants of Polypyrrole and Polyethylene Glycol Obtained by Plasma. J. Mater. Sci. Mater. Med. 2008, 19, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Cruz, G.J.; Mondragón-Lozano, R.; Diaz-Ruiz, A.; Manjarrez, J.; Olayo, R.; Salgado-Ceballos, H.; Olayo, M.G.; Morales, J.; Alvarez-Mejía, L.; Morales, A.; et al. Plasma Polypyrrole Implants Recover Motor Function in Rats after Spinal Cord Transection. J. Mater. Sci. Mater. Med. 2012, 23, 2583–2592. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Mejía, L.; Salgado-Ceballos, H.; Olayo, R.; Cruz, G.J.; Olayo, M.G.; Díaz-Ruiz, A.; Ríos, C.; Mondragón-Lozano, R.; Morales-Guadarrama, A.; Sánchez-Torres, S.; et al. Effect of Pyrrole Implants Synthesized by Different Methods on Spinal Cord Injuries of Rats. Rev. Mex. Ing. Biomed. 2015, 36, 7–21. [Google Scholar]
- Buzoianu-Anguiano, V.; Rivera-Osorio, J.; Orozco-Suárez, S.; Vega-García, A.; García-Vences, E.; Sánchez-Torres, S.; Jiménez-Estrada, I.; Guizar-Sahagún, G.; Mondragon-Caso, J.; Fernández-Valverde, F.; et al. Single vs. Combined Therapeutic Approaches in Rats With Chronic Spinal Cord Injury. Front. Neurol. 2020, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.; Cregg, J.M.; Wang, H.B.; Wendell, D.F.; Oudega, M.; Gilbert, R.J.; McDonald, J.W. Robust CNS Regeneration after Complete Spinal Cord Transection Using Aligned Poly-l-Lactic Acid Microfibers. Biomaterials 2011, 32, 6068–6079. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Ziemba, K.S.; Smith, G.M.; Jin, Y. Evaluation of Cellular Organization and Axonal Regeneration through Linear PLA Foam Implants in Acute and Chronic Spinal Cord Injury. J. Biomed. Mater. Res. Part A 2007, 83, 512–520. [Google Scholar] [CrossRef]
- Saini, P.; Arora, M.; Kumar, M.N.V.R. Poly(Lactic Acid) Blends in Biomedical Applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Ramezani Dana, H.; Ebrahimi, F. Synthesis, Properties, and Applications of Polylactic Acid-Based Polymers. Polym. Eng. Sci. 2023, 63, 22–43. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Alsafar, H.; Hasan, S.W. An Overview of Biodegradable Poly (Lactic Acid) Production from Fermentative Lactic Acid for Biomedical and Bioplastic Applications. Biomass Convers. Biorefin. 2024, 14, 3057–3076. [Google Scholar] [CrossRef]
- Pattanayak, I.; Alex, Y.; Mohanty, S. Advancing Strategies towards the Development of Tissue Engineering Scaffolds: A Review. J. Mater. Sci. 2023, 58, 12847–12898. [Google Scholar] [CrossRef]
- Hanumantharao, S.N.; Rao, S. Multi-Functional Electrospun Nanofibers from Polymer Blends for Scaffold Tissue Engineering. Fibers 2019, 7, 66. [Google Scholar] [CrossRef]
- Osorio-Londoño, D.M.; Godínez-Fernández, J.R.; Acosta-García, M.C.; Morales-Corona, J.; Olayo-González, R.; Morales-Guadarrama, A. Pyrrole Plasma Polymer-Coated Electrospun Scaffolds for Neural Tissue Engineering. Polymers 2021, 13, 3876. [Google Scholar] [CrossRef] [PubMed]
- Younes, H.M.; Kadavil, H.; Ismail, H.M.; Adib, S.A.; Zamani, S.; Alany, R.G.; Al-Kinani, A.A. Overview of Tissue Engineering and Drug Delivery Applications of Reactive Electrospinning and Crosslinking Techniques of Polymeric Nanofibers with Highlights on Their Biocompatibility Testing and Regulatory Aspects. Pharmaceutics 2024, 16, 32. [Google Scholar] [CrossRef]
- Alves, C.M.; Yang, Y.; Marton, D.; Carnes, D.L.; Ong, J.L.; Sylvia, V.L.; Dean, D.D.; Reis, R.L.; Agrawal, C.M. Plasma Surface Modification of Poly(D,L-Lactic Acid) as a Tool to Enhance Protein Adsorption and the Attachment of Different Cell Types. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2008, 87, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.R.; Aleksanderek, I.; Cohen-Adad, J.; Tarmohamed, Z.; Tetreault, L.; Smith, N.; Cadotte, D.W.; Crawley, A.; Ginsberg, H.; Mikulis, D.J.; et al. Translating State-of-the-Art Spinal Cord MRI Techniques to Clinical Use: A Systematic Review of Clinical Studies Utilizing DTI, MT, MWF, MRS, and FMRI. NeuroImage Clin. 2016, 10, 192–238. [Google Scholar] [CrossRef] [PubMed]
- Garrudo, F.F.F.; Mikael, P.E.; Rodrigues, C.A.V.; Udangawa, R.W.; Paradiso, P.; Chapman, C.A.; Hoffman, P.; Colaço, R.; Cabral, J.M.S.; Morgado, J.; et al. Polyaniline-Polycaprolactone Fibers for Neural Applications: Electroconductivity Enhanced by Pseudo-Doping. Mater. Sci. Eng. C 2021, 120, 111680. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Londono, D.M.; Sanchez-Morales, G.S.; Garcia-Garcia, G.; Morales-Guadarrama, A.; Olayo-Gonzalez, R. Pyrrole Plasma Polymer-Coated Fibrillar Scaffold Implant: Pilot Study in Rat Spinal Cord Transection with MRI. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Cancun, Mexico, 1–5 November 2021; pp. 1218–1221. [Google Scholar] [CrossRef]
- Cruz, G.J.; Morales, J.; Olayo, R. Films Obtained by Plasma Polymerization of Pyrrole. Thin Solid Films 1999, 342, 119–126. [Google Scholar] [CrossRef]
- Serratos, I.N.; Olayo, R.; Millán-Pacheco, C.; Morales-Corona, J.; Vicente-Escobar, J.O.; Soto-Estrada, A.M.; Córdoba-Herrera, J.G.; Uribe, O.; Gómez-Quintero, T.; Arroyo-Ornelas, M.Á.; et al. Modeling Integrin and Plasma-Polymerized Pyrrole Interactions: Chemical Diversity Relevance for Cell Regeneration. Sci. Rep. 2019, 9, 7009. [Google Scholar] [CrossRef]
- Van der Meulen, A. The Effects of Switching Light-Dark Regime on the Behavior of Wistar Rats. Master’s Thesis, Utrecht University, Utrecht, The Netherlands, 2014. [Google Scholar]
- Roedel, A.; Storch, C.; Holsboer, F.; Ohl, F. Effects of Light or Dark Phase Testing on Behavioural and Cognitive Performance in DBA Mice. Lab. Anim. 2006, 40, 371–381. [Google Scholar] [CrossRef]
- Bertoglio, L.J.; Carobrez, A.P. Behavioral Profile of Rats Submitted to Session 1-Session 2 in the Elevated plus-Maze during Diurnal/Nocturnal Phases and under Different Illumination Conditions. Behav. Brain Res. 2002, 132, 135–143. [Google Scholar] [CrossRef]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-15400-0. [Google Scholar]
- Kjell, J.; Olson, L. Rat Models of Spinal Cord Injury: From Pathology to Potential Therapies. Dis. Model. Mech. 2016, 9, 1125–1137. [Google Scholar] [CrossRef]
- Kosenko, P.O.; Smolikov, A.B.; Voynov, V.B.; Shaposhnikov, P.D.; Saevskiy, A.I.; Kiroy, V.N. Effect of Xylazine–Tiletamine–Zolazepam on the Local Field Potential of the Rat Olfactory Bulb. Comp. Med. 2020, 70, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Sykes, M.; Matheson, N.A.; Brownjohn, P.W.; Tang, A.D.; Rodger, J.; Shemmeii, J.B.H.; Reynolds, J.N.J. Differences in Motor Evoked Potentials Induced in Rats by Transcranial Magnetic Stimulation under Two Separate Anesthetics: Implications for Plasticity Studies. Front. Neural Circuits 2016, 10, 80. [Google Scholar] [CrossRef]
- Lukovic, D.; Moreno-Manzano, V.; Lopez-Mocholi, E.; Rodriguez-Jiménez, F.J.; Jendelova, P.; Sykova, E.; Oria, M.; Stojkovic, M.; Erceg, S. Complete Rat Spinal Cord Transection as a Faithful Model of Spinal Cord Injury for Translational Cell Transplantation. Sci. Rep. 2015, 5, 9640. [Google Scholar] [CrossRef]
- Ramsey, J.B.G.; Ramer, L.M.; Inskip, J.A.; Alan, N.; Ramer, M.S.; Krassioukov, A.V. Care of Rats with Complete High-Thoracic Spinal Cord Injury. J. Neurotrauma 2010, 27, 1709–1722. [Google Scholar] [CrossRef] [PubMed]
- Sotocinal, S.G.; Sorge, R.E.; Zaloum, A.; Tuttle, A.H.; Martin, L.J.; Wieskopf, J.S.; Mapplebeck, J.C.S.; Wei, P.; Zhan, S.; Zhang, S.; et al. The Rat Grimace Scale: A Partially Automated Method for Quantifying Pain in the Laboratory Rat via Facial Expressions. Mol. Pain 2011, 7, 55. [Google Scholar] [CrossRef]
- Talbot, S.R.; Biernot, S.; Bleich, A.; van Dijk, R.M.; Ernst, L.; Häger, C.; Helgers, S.O.A.; Koegel, B.; Koska, I.; Kuhla, A.; et al. Defining Body-Weight Reduction as a Humane Endpoint: A Critical Appraisal. Lab. Anim. 2020, 54, 99–110. [Google Scholar] [CrossRef]
- Yeh, F.-C.; Verstynen, T.D.; Wang, Y.; Fernández-Miranda, J.C.; Tseng, W.-Y.I. Deterministic Diffusion Fiber Tracking Improved by Quantitative Anisotropy. PLoS ONE 2013, 8, e80713. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.C.; Panesar, S.; Barrios, J.; Fernandes, D.; Abhinav, K.; Meola, A.; Fernandez-Miranda, J.C. Automatic Removal of False Connections in Diffusion MRI Tractography Using Topology-Informed Pruning (TIP). Neurotherapeutics 2019, 16, 52–58. [Google Scholar] [CrossRef]
- de Graaf, R.A. In Vivo NMR Spectroscopy: Principles and Techniques; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2007; ISBN 9780470026700. [Google Scholar]
- Brown, A.R.; Martinez, M. Thoracic Spinal Cord Hemisection Surgery and Open-Field Locomotor Assessment in the Rat. J. Vis. Exp. 2019, 2019, 2–7. [Google Scholar] [CrossRef]
- Basso, M.D.; Beattie, M.S.; Bresnahan, J.C. A Sensitive and Reliable Locomotor Rating Scale for Open Field Testing in Rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Heras-Romero, Y.; Morales-Guadarrama, A.; Santana-Martínez, R.; Ponce, I.; Rincón-Heredia, R.; Poot-Hernández, A.C.; Martínez-Moreno, A.; Urrieta, E.; Bernal-Vicente, B.N.; Campero-Romero, A.N.; et al. Improved Post-Stroke Spontaneous Recovery by Astrocytic Extracellular Vesicles. Mol. Ther. 2021, 30, 798–815. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Jin, W.; Xiao, Z.; Ni, H.; Wang, J.; Kong, J.; Wu, J.; Liang, W.; Chen, L.; Zhao, Y.; et al. The Promotion of Neural Regeneration in an Extreme Rat Spinal Cord Injury Model Using a Collagen Scaffold Containing a Collagen Binding Neuroprotective Protein and an EGFR Neutralizing Antibody. Biomaterials 2010, 31, 9212–9220. [Google Scholar] [CrossRef] [PubMed]
- Antri, M.; Orsal, D.; Barthe, J.Y. Locomotor Recovery in the Chronic Spinal Rat: Effects of Long-Term Treatment with a 5-HT2 Agonist. Eur. J. Neurosci. 2002, 16, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, P.; Griessenauer, C.J.; Cohen-Adad, J.; Rajasekaran, S.; Cauley, K.A.; Shoja, M.M.; Pezeshk, P.; Tubbs, R.S. Spinal Diffusion Tensor Imaging: A Comprehensive Review with Emphasis on Spinal Cord Anatomy and Clinical Applications. Clin. Anat. 2015, 28, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Cecil, K.M. Proton Magnetic Resonance Spectroscopy: Technique for the Neuroradiologist. Neuroimaging Clin. N. Am. 2013, 23, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Herrera, J.J.; Narayana, P.A. Neuronal and Axonal Degeneration in Experimental Spinal Cord Injury: In Vivo Proton Magnetic Resonance Spectroscopy and Histology. J. Neurotrauma 2010, 27, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Erschbamer, M.; Öberg, J.; Westman, E.; Sitnikov, R.; Olson, L.; Spenger, C. 1H-MRS in Spinal Cord Injury: Acute and Chronic Metabolite Alterations in Rat Brain and Lumbar Spinal Cord. Eur. J. Neurosci. 2011, 33, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Kendall, G.S.; Melbourne, A.; Johnson, S.; Price, D.; Bainbridge, A.; Gunny, R.; Huertas-Ceballos, A.; Cady, E.B.; Ourselin, S.; Marlow, N.; et al. White Matter NAA/Cho and Cho/Cr Ratios at MR Spectroscopy Are Predictive of Motor Outcome in Preterm Infants. Radiology 2014, 271, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zeng, W.; Jiang, H.; Ren, X.; Lin, S.; Fan, Y.; Liu, Y.; Zhao, J. Higher Cho/NAA Ratio in Postoperative Peritumoral Edema Zone Is Associated With Earlier Recurrence of Glioblastoma. Front. Neurol. 2020, 11, 592155. [Google Scholar] [CrossRef]
- Guo, J.; Yao, C.; Chen, H.; Zhuang, D.; Tang, W.; Ren, G.; Wang, Y.; Wu, J.; Huang, F.; Zhou, L. The Relationship between Cho/NAA and Glioma Metabolism: Implementation for Margin Delineation of Cerebral Gliomas. Acta Neurochir. 2012, 154, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Abbas, W.A.; Ibrahim, M.E.; El-Naggar, M.; Abass, W.A.; Abdullah, I.H.; Awad, B.I.; Allam, N.K. Recent Advances in the Regenerative Approaches for Traumatic Spinal Cord Injury: Materials Perspective. ACS Biomater. Sci. Eng. 2020, 6, 6490–6509. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Agarwal, T.; Zarepour, A.; Pinelli, F.; Zarrabi, A.; Rossi, F.; Ashrafizadeh, M.; Maleki, A.; Shahbazi, M.A.; Maiti, T.K.; et al. Electroconductive Multi-Functional Polypyrrole Composites for Biomedical Applications. Appl. Mater. Today 2021, 24, 101117. [Google Scholar] [CrossRef]
- Hsu, C.F.; Peng, H.; Basle, C.; Travas-Sejdic, J.; Kilmartin, P.A. ABTS•+ Scavenging Activity of Polypyrrole, Polyaniline and Poly(3,4-Ethylenedioxythiophene). Polym. Int. 2011, 60, 69–77. [Google Scholar] [CrossRef]
- De Winter, F.; Oudega, M.; Lankhorst, A.J.; Hamers, F.P.; Blits, B.; Ruitenberg, M.J.; Pasterkamp, R.J.; Gispen, W.H.; Verhaagen, J. Injury-Induced Class 3 Semaphorin Expression in the Rat Spinal Cord. Exp. Neurol. 2002, 175, 61–75. [Google Scholar] [CrossRef]
- Papa, S.; Mauri, E.; Rossi, F.; Perale, G.; Veglianese, P. Introduction to Spinal Cord Injury as Clinical Pathology. In Spinal Cord Injury (SCI) Repair Strategies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–12. ISBN 9780081028070. [Google Scholar]
- Hou, S.; Rabchevsky, A.G. Autonomic Consequences of Spinal Cord Injury. Compr. Physiol. 2014, 4, 1419–1453. [Google Scholar] [CrossRef] [PubMed]
- Yiu, G.; He, Z. Glial Inhibition of CNS Axon Regeneration. Nat. Rev. Neurosci. 2006, 7, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Harel, N.Y.; Strittmatter, S.M. Can Regenerating Axons Recapitulate Developmental Guidance during Recovery from Spinal Cord Injury? Nat. Rev. Neurosci. 2006, 7, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Rowald, A.; Komi, S.; Demesmaeker, R.; Baaklini, E.; Hernandez-Charpak, S.D.; Paoles, E.; Montanaro, H.; Cassara, A.; Becce, F.; Lloyd, B.; et al. Activity-Dependent Spinal Cord Neuromodulation Rapidly Restores Trunk and Leg Motor Functions after Complete Paralysis. Nat. Med. 2022, 28, 260–271. [Google Scholar] [CrossRef]
- Yu, H.; Yang, S.; Li, H.; Wu, R.; Lai, B.; Zheng, Q. Activating Endogenous Neurogenesis for Spinal Cord Injury Repair: Recent Advances and Future Prospects. Neurospine 2023, 20, 164–180. [Google Scholar] [CrossRef]
- Coyoy-Salgado, A.; Orozco-Barrios, C.; Sánchez-Torres, S.; Olayo, M.G.; Cruz, G.J.; Morales-Corona, J.; Olayo, R.; Diaz-Ruiz, A.; Ríos, C.; Alvarez-Mejia, L.; et al. Gene Expression and Locomotor Recovery in Adult Rats with Spinal Cord Injury and Plasma-Synthesized Polypyrrole/Iodine Application Combined with a Mixed Rehabilitation Scheme. Front. Neurol. 2023, 14, 1124245. [Google Scholar] [CrossRef] [PubMed]
- Vedantam, A.; Jirjis, M.B.; Schmit, B.D.; Wang, M.C.; Ulmer, J.L.; Kurpad, S.N. Diffusion Tensor Imaging of the Spinal Cord: Insights From Animal and Human Studies. Neurosurgery 2014, 74, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moffett, J.; Ross, B.; Arun, P.; Madhavarao, C.; Namboodiri, A. N-Acetylaspartate in the CNS: From Neurodiagnostics to Neurobiology. Prog. Neurobiol. 2007, 81, 89–131. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Dai, Y.J.; Chen, G.; Cui, S. Sen Dissecting the Dual Role of the Glial Scar and Scar-Forming Astrocytes in Spinal Cord Injury. Front. Cell. Neurosci. 2020, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.L.; Zhang, Y.; Ning, B. Reactive Astrocytes in Central Nervous System Injury: Subgroup and Potential Therapy. Front. Cell. Neurosci. 2021, 15, 792764. [Google Scholar] [CrossRef] [PubMed]
- Vipin, A.; Thow, X.Y.; Mir, H.; Kortelainen, J.; Manivannan, J.; Al-Nashash, H.; All, A.H. Natural Progression of Spinal Cord Transection Injury and Reorganization of Neural Pathways. J. Neurotrauma 2016, 33, 2191–2201. [Google Scholar] [CrossRef]
- Aguilar, J.; Humanes-Valera, D.; Alonso-Calviño, E.; Yague, J.G.; Moxon, K.A.; Oliviero, A.; Foffani, G. Spinal Cord Injury Immediately Changes the State of the Brain. J. Neurosci. 2010, 30, 7528–7537. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Ortega, M.; Ortega, M.; Morales, J.; Olayo, M.G.; Cruz, G.J.; Olayo, R. Core-Shell Polypyrrole Nanoparticles Obtained by Atmospheric Pressure Plasma Polymerization. Polym. Int. 2014, 63, 2023–2029. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, J.; Guo, Y. Synthesis of Intrinsic Fluorescent Polypyrrole Nanoparticles by Atmospheric Pressure Plasma Polymerization. Appl. Surf. Sci. 2009, 255, 6924–6929. [Google Scholar] [CrossRef]
- Du, B.L.; Zeng, C.G.; Zhang, W.; Quan, D.P.; Ling, E.A.; Zeng, Y.S. A Comparative Study of Gelatin Sponge Scaffolds and PLGA Scaffolds Transplanted to Completely Transected Spinal Cord of Rat. J. Biomed. Mater. Res.-Part A 2014, 102, 1715–1725. [Google Scholar] [CrossRef]
- Li, X.; Liu, D.; Xiao, Z.; Zhao, Y.; Han, S.; Chen, B.; Dai, J. Scaffold-Facilitated Locomotor Improvement Post Complete Spinal Cord Injury: Motor Axon Regeneration versus Endogenous Neuronal Relay Formation. Biomaterials 2019, 197, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, H. Research Progress of Endogenous Neural Stem Cells in Spinal Cord Injury. Ibrain 2022, 8, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Li, X.; Xiao, Z.; Zhao, Y.; Liang, H.; Wang, B.; Han, S.; Li, X.; Xu, B.; Wang, N.; et al. A Modified Collagen Scaffold Facilitates Endogenous Neurogenesis for Acute Spinal Cord Injury Repair. Acta Biomater. 2017, 51, 304–316. [Google Scholar] [CrossRef]
- Colín, E.; Olayo, M.G.; Cruz, G.J.; Carapia, L.; Morales, J.; Olayo, R. Affinity of Amine-Functionalized Plasma Polymers with Ionic Solutions Similar to Those in the Human Body. Prog. Org. Coat. 2009, 64, 322–326. [Google Scholar] [CrossRef]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osorio-Londoño, D.; Heras-Romero, Y.; Tovar-y-Romo, L.B.; Olayo-González, R.; Morales-Guadarrama, A. Improved Recovery of Complete Spinal Cord Transection by a Plasma-Modified Fibrillar Scaffold. Polymers 2024, 16, 1133. https://doi.org/10.3390/polym16081133
Osorio-Londoño D, Heras-Romero Y, Tovar-y-Romo LB, Olayo-González R, Morales-Guadarrama A. Improved Recovery of Complete Spinal Cord Transection by a Plasma-Modified Fibrillar Scaffold. Polymers. 2024; 16(8):1133. https://doi.org/10.3390/polym16081133
Chicago/Turabian StyleOsorio-Londoño, Diana, Yessica Heras-Romero, Luis B. Tovar-y-Romo, Roberto Olayo-González, and Axayácatl Morales-Guadarrama. 2024. "Improved Recovery of Complete Spinal Cord Transection by a Plasma-Modified Fibrillar Scaffold" Polymers 16, no. 8: 1133. https://doi.org/10.3390/polym16081133
APA StyleOsorio-Londoño, D., Heras-Romero, Y., Tovar-y-Romo, L. B., Olayo-González, R., & Morales-Guadarrama, A. (2024). Improved Recovery of Complete Spinal Cord Transection by a Plasma-Modified Fibrillar Scaffold. Polymers, 16(8), 1133. https://doi.org/10.3390/polym16081133