Cure Kinetics and Thermal Decomposition Behavior of Novel Phenylacetylene-Capped Polyimide Resins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Section
2.1.1. Experimental Materials
2.1.2. Preparation of PI Resins
2.2. Characterization
2.3. Molecular Dynamics Simulation
3. Results
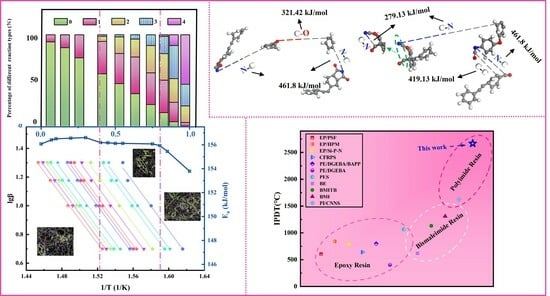
3.1. Curing Behavior and Curing Kinetics
- (1)
- Determination of apparent activation energy and reaction order by multiple heating rate method
- (2)
- Determination of kinetic parameters of the curing reaction by the equal conversion method
3.2. Molecular Dynamics Simulation of Curing Reaction Process
3.3. Thermal Degradation Behavior
3.4. Thermal Stability and the Activation Energy of Thermal Decomposition
3.5. Molecular Dynamics Simulation of Thermal Decomposition Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.-J.; Zheng, M.-S.; Chen, G.; Dang, Z.-M.; Zha, J.-W. High-temperature polyimide dielectric materials for energy storage: Theory, design, preparation and properties. Energy Environ. Sci. 2022, 15, 56–81. [Google Scholar] [CrossRef]
- Zhang, J.; Fox, B.L. Manufacturing Influence on the Delamination Fracture Behavior of the T800H/3900-2 Carbon Fiber Reinforced Polymer Composites. Mater. Manuf. Process. 2007, 22, 768–772. [Google Scholar] [CrossRef]
- Qi, S.-B.; Cui, X.; Liu, Y.-T.; Li, X.; Li, J.-H.; Zheng, R.-R.; Ruan, X.-H.; Wang, C.; Guo, L.-Y. Preparation and properties of electrochromic polyimide hybrid materials containing graphene oxide. Mater. Today Commun. 2024, 38, 107685. [Google Scholar] [CrossRef]
- Pan, Z.; Han, S.; Wang, J.; Qi, S.; Tian, G.; Wu, D. Polyimide fabric-reinforced polyimide matrix composites with excellent thermal, mechanical, and dielectric properties. High Perform. Polym. 2020, 32, 1085–1093. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, X.; Zheng, F.; Lu, X.; Lu, Q. Synthesis of highly transparent and heat-resistant polyimides containing bulky pendant moieties. Polym. Int. 2019, 68, 1186–1193. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z.; Ji, H.; Wang, J.; Lu, K.; Xu, G.; Xiao, Y.; Ding, F. Mechanically Robust Nanoporous Polyimide/Silica Aerogels for Thermal Superinsulation of Aircraft. ACS Appl. Nano Mater. 2023, 6, 7269–7279. [Google Scholar] [CrossRef]
- Rose, N.; Le Bras, M.; Bourbigot, S.; Delobel, R. Thermal oxidative degradation of epoxy resins: Evaluation of their heat re-sistance using invariant kinetic parameters. Polym. Degrad. Stab. 1994, 45, 387–397. [Google Scholar] [CrossRef]
- Gu, A.; Liang, G.; Lan, L. A high-performance bismaleimide resin with good processing characteristics. J. Appl. Polym. Sci. 1996, 62, 799–803. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Hu, J. Recent advances in the development of aerospace materials. Prog. Aerosp. Sci. 2018, 97, 22–34. [Google Scholar] [CrossRef]
- Sun, G.H.; Xin, Z.; Wu, D.Z.; Hou, L.L. Research Progress in High⁃performance Polyimide Composites. China Plast. 2021, 35, 147. [Google Scholar]
- Li, C.Q.; Wang, Y.X.; Yin, Y.Y.; Li, Y.Y.; Li, J.H.; Sun, D.L.; Lu, J.B.; Zhang, G.P.; Sun, R. A comprehensive study of pyra-zine-contained and low-temperature curable polyimide. Polymer 2021, 228, 123963. [Google Scholar] [CrossRef]
- Wang, T.; Jiao, Y.; Mi, Z.; Li, J.; Wang, D.; Zhao, X.; Zhou, H.; Chen, C. PEEK composites with polyimide sizing SCF as reinforcement: Preparation, characterization, and mechanical properties. High Perform. Polym. 2020, 32, 383–393. [Google Scholar] [CrossRef]
- Chen, D.S.; Chen, C.H.; Whang, W.T.; Su, C.-W. Interpenetration networked polyimide–epoxy copolymer under kinetic and ther-modynamic control for anticorrosion coating. Polymers 2023, 15, 243. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Yang, J.; Zhang, C. Phenylethynyl terminated polyimide resin/carbon fiber composite catalytic cured at 300 °C and its performance. J. Adhes. Sci. Technol. 2021, 35, 2652–2663. [Google Scholar] [CrossRef]
- Cheng, S.L.; Han, J.H.; Wang, X.; Yuan, K.Y.; Jian, X.G.; Wang, J.Y. Oxidatively stable thermosets derived from thermal co-polymerization of acetylene-terminated imide monomer with an acetylenic monomer containing carborane. Polymer 2017, 115, 96–105. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L.; Lin, J.; Du, L. Thermal curing mechanism of acetylene-terminated polyimides: A combination of density functional theory computation and microkinetic analysis. Polymer 2021, 218, 123529. [Google Scholar] [CrossRef]
- Khazaka, R.; Locatelli, M.L.; Diaham, S.; Bidan, P. Effects of mechanical stresses, thickness and atmosphere on aging of pol-yimide thin films at high temperature. Polym. Degrad. Stab. 2013, 98, 361–367. [Google Scholar] [CrossRef]
- Kim, H.; Park, J.; Bak, S.; Park, J.; Byun, C.; Oh, C.; Kim, B.S.; Han, C.; Yoo, J.; Kim, D.; et al. Effects of polyimide curing on image sticking behaviors of flexible displays. Sci. Rep. 2021, 11, 21805. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Li, W.; Ren, R.; Wang, J. Isothermal curing analysis and properties of ultra heat-resistant polyimide composites by DMA method. J. Appl. Polym. Sci. 2023, 140, e54056. [Google Scholar] [CrossRef]
- Wisanrakkit, G.; Gillham, J.K. The glass transition temperature (Tg) as an index of chemical conversion for a high-Tg amine/epoxy system: Chemical and diffusion-controlled reaction kinetics. J. Appl. Polym. Sci. 1990, 41, 2885–2929. [Google Scholar] [CrossRef]
- Xiong, X.; Chen, P.; Zhang, J.; Yu, Q.; Wang, B. Preparation and properties of high performance phthalide-containing bismaleimide modified epoxy matrices. J. Appl. Polym. Sci. 2011, 121, 3122–3130. [Google Scholar] [CrossRef]
- Musto, P.; Martuscelli, E.; Ragosta, G.; Russo, P.; Scarinzi, G. FTIR spectroscopy and physical properties of an epoxy/bismaleimide IPN system. J. Mater. Sci. 1998, 33, 4595–4601. [Google Scholar] [CrossRef]
- Musto, P.; Martuscelli, E.; Ragosta, G.; Russo, P.; Scarinzi, G. An interpenetrated system based on a tetrafunctional epoxy resin and a thermosetting bismaleimide: Structure–properties correlation. J. Appl. Polym. Sci. 1998, 69, 1029–1042. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Matečić Mušanić, S.; Fiamengo Houra, I.; SućeSkA, M. Applicability of non-isothermal DSC and Ozawa method for studying kinetics of double base propellant decomposition. Cent. Eur. J. Energetic Mater. 2010, 7, 233–251. [Google Scholar]
- Santos, J.G.; Conceicao, M.M.; Trindade, M.F.; Araujo, A.S.; Fernandes, V.J., Jr.; Souza, A.G. Kinetic study of dipivaloylmethane by Ozawa method. J. Therm. Anal. Calorim. 2004, 75, 591–597. [Google Scholar] [CrossRef]
- Crane, L.W.; Dynes, P.J.; Kaelble, D.H. Analysis of curing kinetics in polymer composites. J. Polym. Sci. Polym. Lett. Ed. 1973, 11, 533–540. [Google Scholar] [CrossRef]
- Fang, X.M.; Xie, X.Q.; Simone, C.D.; Stevens, M.P.; Scola, D.A. A solid-state 13C NMR study of the cure of 13C-labeled phe-nylethynyl end-capped polyimides. Macromolecules 2000, 33, 1671–1681. [Google Scholar] [CrossRef]
- Riccardi, C.C.; Adabbo, H.E.; Williams, R.J.J. Curing reaction of epoxy resins with diamines. J. Appl. Polym. Sci. 1984, 29, 2481–2492. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Doyle, C.D. Series Approximations to the Equation of Thermogravimetric Data. Nature 1965, 207, 290–291. [Google Scholar] [CrossRef]
- Starink, M. A new method for the derivation of activation energies from experiments performed at constant heating rate. Thermochim. Acta 1996, 288, 97–104. [Google Scholar] [CrossRef]
- Lauver, R.W. Kinetics of imidization and crosslinking in PMR polyimide resin. J. Polym. Sci. Polym. Chem. Ed. 1979, 17, 2529–2539. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Kim, K.-W.; Kim, B.-J. Thermal and Mechanical Characterization of Epoxy/Polyimide Blends via Postcuring Process. Polymers 2023, 15, 1072. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Kang, M.-J.; Kim, S.-H.; Rhee, K.Y.; Lee, J.-H.; Park, S.-J. Roles of Small Polyetherimide Moieties on Thermal Stability and Fracture Toughness of Epoxy Blends. Polymers 2021, 13, 3310. [Google Scholar] [CrossRef]
- Xu, M.; Lei, Y.; Ren, D.; Chen, L.; Li, K.; Liu, X. Thermal stability of allyl-functional phthalonitriles-containing benzoxa-zine/bismaleimide copolymers and their improved mechanical properties. Polymers 2018, 10, 596. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Kim, H.-C. Thermal stability and toughening of epoxy resin with polysulfone resin. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 121–128. [Google Scholar] [CrossRef]
- Wu, C.-S.; Liu, Y.-L.; Hsu, K.-Y. Maleimide-epoxy resins: Preparation, thermal properties, and flame retardance. Polymer 2003, 44, 565–573. [Google Scholar] [CrossRef]
- Chiang, C.-L.; Chang, R.-C.; Chiu, Y.-C. Thermal stability and degradation kinetics of novel organic/inorganic epoxy hybrid containing nitrogen/silicon/phosphorus by sol–gel method. Thermochim. Acta 2007, 453, 97–104. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, D.K.; Kim, B.S.; An, K.Y.; Park, S.J.; Rhee, K.Y.; Kim, B.J. Cure behaviors and mechanical properties of carbon fiber-reinforced nylon6/epoxy blended matrix composites. Compos. Part B Eng. 2017, 112, 15–21. [Google Scholar] [CrossRef]
- Xiong, X.; Zhou, L.; Ren, R.; Liu, S.; Chen, P. The thermal decomposition behavior and kinetics of epoxy resins cured with a novel phthalide-containing aromatic diamine. Polym. Test. 2018, 68, 46–52. [Google Scholar] [CrossRef]
- Lee, S.E.; Jeong, E.; Lee, M.Y.; Lee, M.K.; Lee, Y.S. Improvement of the mechanical and thermal properties of polyethersul-fone-modified epoxy composites. J. Ind. Eng. Chem. 2016, 33, 73–79. [Google Scholar]
- Varma, I.; Sharma, S. Curing of bismaleimides—I. Effect of amines on the curing behaviour and thermal stability. Eur. Polym. J. 1984, 20, 1101–1105. [Google Scholar] [CrossRef]
- Zhou, B.X.; Huang, Y.J.; Zhang, X.H.; Fu, Z.S.; Qi, G.R. Thermal properties of an epoxy cresol-formaldehyde novo-lac/diaminodiphenyl sulfone system modified by bismaleimide containing tetramethylbiphenyl and aromatic ether struc-tures. Polym. Eng. Sci. 2009, 49, 1525–1532. [Google Scholar] [CrossRef]
- Liu, Y.L.; Chen, Y.J. Novel thermosetting resins based on 4-(N-maleimidophenyl) glycidylether: II. Bismaleimides and po-lybismaleimides. Polymer 2004, 45, 1797–1804. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Ding, X.; Zhang, P.; Shu, M.; Zhang, Q.; Gong, Y.; Zheng, K.; Tian, X. Imidization-induced carbon nitride nanosheets orientation towards highly thermally conductive polyimide film with superior flexibility and electrical insulation. Compos. Part B Eng. 2020, 199, 108267. [Google Scholar] [CrossRef]
















| β °C/min | Ti (°C) | Tp (°C) | Tf (°C) |
|---|---|---|---|
| 5 | 346.8 | 372.7 | 388.1 |
| 10 | 359.5 | 391.1 | 405.1 |
| 15 | 362.7 | 398.0 | 420.2 |
| 20 | 369.5 | 403.1 | 423.8 |
| State | T5% (°C) | T20% (°C) | Tmax (°C) | RW (%) | IPDT | ||
|---|---|---|---|---|---|---|---|
| A* | K* | T (°C) | |||||
| PI resins | 537 | 612 | 573 | 64.3 | 0.89 | 3.61 | 2127.7 |
| cured PI resins | 571 | 631 | 605 | 70.6 | 0.91 | 4.52 | 2667.9 |
| State | Ea1 | R2 | Ea2 | R2 |
|---|---|---|---|---|
| PI resins | 136.4 | 0.988 | 60.7 | 0.982 |
| Cured PI resins | 193.2 | 0.998 | 42.3 | 0.976 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, X.; Guan, H.; Li, B.; Yang, S.; Li, W.; Ren, R.; Wang, J.; Chen, P. Cure Kinetics and Thermal Decomposition Behavior of Novel Phenylacetylene-Capped Polyimide Resins. Polymers 2024, 16, 1149. https://doi.org/10.3390/polym16081149
Xiong X, Guan H, Li B, Yang S, Li W, Ren R, Wang J, Chen P. Cure Kinetics and Thermal Decomposition Behavior of Novel Phenylacetylene-Capped Polyimide Resins. Polymers. 2024; 16(8):1149. https://doi.org/10.3390/polym16081149
Chicago/Turabian StyleXiong, Xuhai, Hongyu Guan, Baiyu Li, Shuai Yang, Wenqiang Li, Rong Ren, Jing Wang, and Ping Chen. 2024. "Cure Kinetics and Thermal Decomposition Behavior of Novel Phenylacetylene-Capped Polyimide Resins" Polymers 16, no. 8: 1149. https://doi.org/10.3390/polym16081149
APA StyleXiong, X., Guan, H., Li, B., Yang, S., Li, W., Ren, R., Wang, J., & Chen, P. (2024). Cure Kinetics and Thermal Decomposition Behavior of Novel Phenylacetylene-Capped Polyimide Resins. Polymers, 16(8), 1149. https://doi.org/10.3390/polym16081149