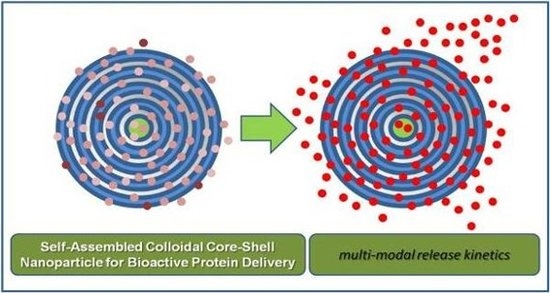
Bio-Inspired/-Functional Colloidal Core-Shell Polymeric-Based NanoSystems: Technology Promise in Tissue Engineering, Bioimaging and NanoMedicine
Abstract
:1. Introduction
2. Polymeric-Based Core-Shell Colloids



3. Bio-Functionalized Core-Shell Designed Nanomaterials and Nanosystems
4. Bio-Inspired Polymeric-Based Core-Shell Nanoparticulate Systems
5. Bio-Inspired Surface Chemistry and Bio-Functional Tailoring
6. Biomedical Applications of Core-Shell Polymeric-Based Nanostructures
6.1. Bio-Sensing in Diabetes Mellitus: Glucose Level Monitoring
6.2. Bio-Imaging: Biological and Cell Membrane/Intra-Cellular Labelling
7. Associated Safety Issues
8. Nanoncology
9. Drug- and Gene-Delivery in Tissue Engineering and Regenerative Medicine
9.1. Bio-Inspired Polymer-Based Drug Delivery Systems: The L-b-L Self-Assembly

9.2. Synthetic and Composite Drug Delivery Systems: Bio-Functional Nano-Shells
10. Miscellaneous Applications of Core-Shell Nanostructures
11. Prospects
Abbreviations
| AL | alginate |
| BMP-7/OP-1 | bone morphogenetic protein -7/Osteogenic protein-1 |
| CdSe | cadmium selenide |
| ZnS | zinc sulfide |
| CH | chitosan |
| GOD | glucose oxidase |
| HA | hyaluronic acid |
| IgG | Immunoglobulin G |
| L | liposome |
| L-b-L | layer-by-layer self-assembly technique |
| MPS | mononuclear phagocyte system |
| MRI | magnetic resonance imaging |
| MW | molecular weight |
| nIR | near infra-red |
| NZW | New Zealand White |
| PAC | poly aluminum chloride (cationic) |
| PCL | poly(ε-caprolactone) |
| PEG | polyethylene glycol |
| PEO | polyethylene oxide |
| PGA | polyglycolic acid |
| PLA | polylactic acid |
| PLGA | poly(lactic-co-glycolic acid) |
| PLLA | poly(l-lactide) |
| PVA | poly (vinyl alcohol) |
| PVP | polyvinylpyrrolidone |
| QDs | Quantum Dots |
| RES | reticulo-endothelial system |
| rh | recombinant human |
Acknowledgments
References
- Sahoo, S.K.; Labhasetwar, V. Nanotech approaches to drug delivery and imaging. Drug Discov. Today 2003, 8, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, J.L.; Yi, X.; Quan, L.; Kabanov, A.V. Novel Nanomaterials for Clinical Neuroscience. J. Neuroimmune Pharmacol. 2008, 3, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, J.J. What is nanotechnology? Nanotechnol. Percept. 2005, 1, 3–17. [Google Scholar] [CrossRef]
- Murray, C.B.; Kagan, C.R.; Bawendi, M.G. Synthesis and characterisation of monodisperse nanocrystals and close-packed nanocrystals assemblies. Annu. Rev. Mater. Sci. 2000, 30, 545–610. [Google Scholar] [CrossRef]
- Whitesides, G.M. The 'right' size in Nanobiotechnology. Nat. Biotech. 2003, 2, 1161–1165. [Google Scholar] [CrossRef]
- Parak, W.J.; Gerion, D.; Pellegrino, T.; Zanchet, D.; Micheel, C.; Williams, C.S.; Boudreau, R.; Le Gros, M.A.; Larabell, C.A.; Alivisatos, A.P. Biological applications of colloidal nanocrystals. Nanotechnology 2003, 14, R15–R27. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef]
- Haidar, Z.S.; Hamdy, R.C.; Tabrizian, M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A: Current challenges in BMP delivery. Biotechnol. Lett. 2009, 31, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Haidar, Z.S.; Hamdy, R.C.; Tabrizian, M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part B: Delivery systems for BMPs in orthopaedic and craniofacial tissue engineering. Biotechnol. Lett. 2009, 31, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Wang, Y.; Powell, R.; Chan, P. Polymeric core-shell nanoparticles for therapeutics. Clin. Exp. Pharm. Physio. 2006, 33, 557–562. [Google Scholar] [CrossRef]
- Lee, W.F.; Cheng, T.S. Synthesis and drug-release behavior of porous biodegradable amphiphilic co-polymeric hydrogels. J. Biomater. Sci. Polym. Ed. 2009, 20, 2023–2037. [Google Scholar] [CrossRef] [PubMed]
- Schärtl, W. Current directions in core–shell nanoparticle design. Nanoscale 2010, 2, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Pothayee, N.; Seleem, M.N.; Tyler, R.D., Jr.; Brenseke, B.; Sriranganathan, N.; Riffle, J.S.; Kasimanickam, R. Antibacterial efficacy of core-shell nanostructures encapsulating gentamicin against an in vivo intracellular Salmonella model. Int. J. Nanomed. 2009, 4, 289–297. [Google Scholar] [CrossRef]
- Ydens, I.; Degee, P.; Nouvel, C.; Dellacherie, E.; Six, J.L.; Dubois, P. Surfactant-free’ stable nanoparticles from biodegradable and amphiphilic poly(ε-caprolactone)-grafted dextran copolymers. e-Polymers 2005, 46, 1–11. [Google Scholar]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloid. Surface. B 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Finne-Wistrand, A.; Albertsson, A.C. The use of polymer design in resorbable colloids. Annu. Rev. Mater. Res. 2006, 36, 369–395. [Google Scholar] [CrossRef]
- Boscovic, B.O. Carbon nanotubes and nanofibres. Nanotechnol. Percept. 2007, 3, 141–158. [Google Scholar]
- Zandonella, C. The tiny toolkit. Nature 2003, 423, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, L. Commercializing nanotechnology. Nat. Biotech. 2003, 21, 1137–1143. [Google Scholar] [CrossRef]
- Haidar, Z.S.; Hamdy, R.C.; Tabrizian, M. Protein release kinetics for core-shell hybrid nanoparticles based on the layer-by-layer assembly of alginate and chitosan on liposomes. Biomaterials 2008, 29, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Sounderya, N.; Zhang, Y. Use of Core/Shell Structured Nanoparticles for Biomedical Applications. Recent Pat. Biomed. Eng. 2008, 1, 34–42. [Google Scholar] [CrossRef]
- Burda, C.; Chen, X.; Narayana, R.; El Sayed, M.A. Chemistry and Properties of Nanocrystals of Different Shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Angelatos, A.S.; Katagiri, K.; Caruso, F. Bioinspired colloidal systems via layer-by-layer assembly. Soft Matter. 2006, 2, 18–23. [Google Scholar]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2002, 54, 631–651. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, S.; Bikiaris, D. Novel self-assembled core–shell nanoparticles based on crystalline amorphous moieties of aliphatic copolyesters for efficient controlled drug release. J. Control. Release 2009, 138, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Schreder, B.; Schmidt, T.; Ptatschek, V.; Spanhel, L.; Materny, A.; Kiefer, W. Raman characterization of CdTe/CdS-core-shell-clusters in colloids and films. J. Cryst. Growth 2000, 214, 782–786. [Google Scholar] [CrossRef]
- Chu, M.Q.; Cheng, D.L.; Zhu, J. Preparation of quantum dotcoated magnetic polystyrene nanospheres for cancer cell labelling and separation. Nanotecnology 2006, 17, 3268–3273. [Google Scholar] [CrossRef]
- Zhou, M.; Gho, I. Quantum dots and peptides: A bright future together. Pept. Sci. 2006, 88, 325–339. [Google Scholar] [CrossRef]
- Sandros, M.G.; Shete, V.; Benson, D.E. Selective, reversible, reagentless maltose biosensing with core–shell semiconducting nanoparticles. Analyst 2006, 131, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.Y.; Kim, M.J.; Kim, S.T.; Oh, K.S.; Yuk, S.H.; Lee, S. Size characterization of drug-loaded polymeric core/shell nanoparticles using asymmetrical flow field-flow fractionation. Anal. Bioanal. Chem. 2008, 390, 2183–2188. [Google Scholar]
- Chilcott, J.; Lloyd Jones, M.; Wilkinson, A. Docetaxel for the adjuvant treatment of early node-positive breast cancer: a single technology appraisal. Health Technol. Assess. 2009, 13, 7–11. [Google Scholar] [PubMed]
- Wei, Z.; Hao, J.; Yuan, S.; Li, Y.; Juan, W.; Sha, X.; Fang, X. Paclitaxel-loaded Pluronic P123/F127 mixed polymeric micelles: formulation, optimization and in vitro characterization. Int. J. Pharm. 2009, 376, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Sethuraman, V.A.; Bae, Y.H. TAT peptide-based micelle system for potential active targeting of anti-cancer agents to acidic solid tumors. J. Contol. Release 2007, 118, 216–224. [Google Scholar] [CrossRef]
- Borini, S.; D'Auria, S.; Rossi, M.; Rossi, A.M. Writing 3D protein nanopatterns onto a silicon nanosponge. Lab Chip 2005, 5, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: from controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Kojima, C. Design of stimuli-responsive dendrimers. Expert Opin. Drug Deliv. 2010, 7, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.M.; Mano, J.F. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int. J. Biol. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Kuroda, K. Layer-by-layer assembly of imogolite nanotubes and polyelectrolytes into core-shell particles and their conversion to hierarchically porous spheres. Sci. Technol. Adv. Mater. 2008, 9, 250–218. [Google Scholar]
- Srivastava, S.; Kotov, N.A. Composite Layer-by-Layer (LBL) assembly with inorganic nanoparticles and nanowires. Account Chem. Res. 2008, 12, 1831–1841. [Google Scholar] [CrossRef]
- Owens, D.E., III; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Preston, T.C.; Signorell, R. Growth and optical properties of gold nanoshells prior to the formation of a continuous metallic layer. ACS Nano. 2009, 3, 3696–3706. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Ye, M.; Park, K. Biodegradable polymers for microencapsulation of drugs. Molecules 2005, 10, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Azzam, T.; Eisenberg, A. Monolayer-protected gold nanoparticles by the self-assembly of micellar poly(ethylene oxide)-b-poly(epsilon-caprolactone) block copolymer. Langmuir 2007, 23, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Ramzi, A.; Rijcken, C.J.; Veldhuis, T.F.; Schwahn, D.; Hennink, W.E.; van Nostrum, C.F. Core-shell structure of degradable, thermosensitive polymeric micelles studied by small-angle neutron scattering. J. Phys. Chem. B 2008, 112, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Haidar, Z.S.; Tabrizian, M.; Hamdy, R.C. A hybrid rhOP-1 delivery system enhances new bone regeneration and consolidation in a rabbit model of distraction osteogenesis. Growth Factors 2010, 28, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Haidar, Z.S.; Hamdy, R.C.; Tabrizian, M. Biocompatibility and safety of a hybrid core-shell nanoparticulate OP-1 delivery system intramuscularly administered in rats. Biomaterials 2010, 31, 2746–2754. [Google Scholar] [CrossRef] [PubMed]
- Haidar, Z.S.; Azari, F.; Hamdy, R.C.; Tabrizian, M. Modulated release of OP-1 and enhanced preosteoblast differentiation using a core-shell nanoparticulate system. J. Biomed. Mater. Res. A 2009, 91, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Misra, R.D.K. Magnetic drug-targeting carrier encapsulated with thermosensitive smart polymer: Core–shell nanoparticle carrier and drug release response. Acta Biomater. 2007, 3, 838–850. [Google Scholar] [CrossRef] [PubMed]
- Surendiran, A.; Sandhiya, S.; Pradhan, S.C.; Adithan, C. Novel applications of nanotechnology in medicine. Indian J. Med. Res. 2009, 130, 689–701. [Google Scholar] [PubMed]
- Santander-Ortega, M.J.; Bastos-González, D.; Ortega-Vinuesa, J.L. Electrophoretic mobility and colloidal stability of PLGA particles coated with IgG. Colloid. Surface B 2007, 60, 80–88. [Google Scholar] [CrossRef]
- Sant, S.; Poulin, S.; Hildgen, P. Effect of polymer architecture on surface properties, plasma protein adsorption, and cellular interactions of pegylated nanoparticles. J. Biomed. Mater. Res. A 2008, 87, 885–895. [Google Scholar] [CrossRef] [PubMed]
- West, J.L. Biofunctional Polymers. Encycl Biomater. Biomed. Eng. 2004, 1, 89–95. [Google Scholar]
- Wissink, M.J.B.; Beernink, R.; Poot, A.A.; Engbers, G.H.M.; Beugling, T.; van Aken, W.G.; Feijen, J. Improved endothelialization of vascular grafts by local release of growth factor from heparinized collagen matrices. J. Control. Release 2000, 64, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Mann, B.K.; Schmedlen, R.H.; West, J.L. Tethered TGFbeta increases extracellular matrix production of vascular smooth muscle cells. Biomaterials 2001, 22, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.J.; West, J.L. Vascularization of Engineered Tissues: Approaches to Promote Angiogenesis in Biomaterials. Curr. Top. Med. Chem. 2008, 8, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Klonoff, D.C. Technological advances in the treatment of diabetes mellitus: better bioengineering begets benefits in glucose measurement, the artificial pancreas, and insulin delivery. Pediatr. Endocrinol. Rev. 2003, 2, 94–100. [Google Scholar]
- Pickup, J.C.; Zhi, Z.L.; Khan, F.; Saxl, T.; Birch, D.J. Nanomedicine and its potential in diabetes research and practice. Diabetes Metab. Res. Rev. 2008, 24, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Orth, A.; Le, B.; Menchavez, P.; Miller, L. Performance Analysis of the OneTouch(R) UltraVue Blood Glucose Monitoring System. J. Diabetes Sci. Technol. 2009, 3, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Musameh, M. Enzyme-dispersed carbon-nanotube electrodes: a needle microsensor for monitoring glucose. Analyst 2003, 128, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Chen, J. Direct electron transfer of glucose oxidase promoted by carbon nanotubes. Anal. Biochem. 2004, 332, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, H.; Park, J.S.; Choi, H.; Han, K.Y.; Seo, H.S.; Ahn, K.Y.; Han, S.S.; Cho, Y.; Lee, K.H.; Lee, J. A novel approach to ultrasensitive diagnosis using supramolecular protein nanoparticles. FASEB J. 2007, 21, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.D.; Rhee, J.I. Use of CdSe/ZnS core-shell quantum dots as energy transfer donors in sensing glucose. Talanta 2007, 73, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Véronique, L.V.; Anclaa, C.; Catargic, B.; Ravaine, V. Glucose-responsive microgels with a core–shell structure. J. Colloid. Interface Sci. 2008, 327, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Ramesan, R.M.; Sharma, C.P. Challenges and advances in nanoparticle-based oral insulin delivery. Expert Rev. Med. Devices 2009, 6, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Scodeller, P.; Flexer, V.; Szamocki, R.; Calvo, E.J.; Tognalli, N.; Troiani, H.; Fainstein, A. Wired-Enzyme Core-Shell Au Nanoparticle Biosensor. J. Am. Chem. Soc. 2008, 130, 12690–12697. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.W.; Hsu, S.H.; Wang, J.J.; Tsai, J.S.; Lin, K.J.; Wey, S.P.; Chen, F.R.; Lai, C.H.; Yen, T.C.; Sung, H.W. The characteristics, biodistribution, magnetic resonance imaging and biodegradability of superparamagnetic core-shell nanoparticles. Biomaterials 2010, 31, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Purushotham, S.; Chang, P.E.; Rumpel, H.; Kee, I.H.; Ng, R.T.; Chow, P.K.; Tan, C.K.; Ramanujan, R.V. Thermoresponsive core-shell magnetic nanoparticles for combined modalities of cancer therapy. Nanotechnology 2009, 20, 305101. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Liu, W.; Liu, F.; Nasr, K.; Misra, P.; Bawendi, M.G.; Frangioni, J.V. Design considerations for tumour-targeted nanoparticles. Nat. Nanotech. 2010, 5, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Duan, D.; Publicover, N.G. Magnetic bead based assay for C-reactive protein using quantum-dot fluorescence labeling and immunoaffinity separation. Analyst 2010, 135, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Sha, M.Y.; Wong, E.Y.; Uphoff, J.; Xu, Y.; Treadway, J.A.; Truong, A.; O'Brien, E.; Asquith, S.; Stubbins, M.; Spurr, N.K.; Lai, E.H.; Mahoney, W. Multiplexed SNP genotyping using the Qbead system: A quantum dot-encoded microsphere-based assay. Nucl. Acid. Res. 2003, 31, 43. [Google Scholar] [CrossRef]
- Behrendt, M.; Sandros, M.G.; McKinney, R.A.; McDonald, K.; Przybytkowski, E.; Tabrizian, M.; Maysinger, D. Imaging and organelle distribution of fluorescent InGaP/ZnS nanoparticles in glial cells. Nanomedicine (London) 2009, 4, 747–761. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, H.Y.; Kim, J.; Ryu, J.; Hong, S.; Han, S.J.; Song, R. Western blot analysis using metal-nitrilotriacetate conjugated CdSe/ZnS quantum dots. Anal. Biochem. 2008, 379, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Shiohara, A.; Hanada, S.; Prabakar, S.; Fujioka, K.; Lim, T.H.; Yamamoto, K.; Northcote, P.T.; Tilley, R.D. Chemical reactions on surface molecules attached to silicon quantum dots. J. Am. Chem. Soc. 2010, 132, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sachdev, D.; Wang, C.; Hubel, A.; Gaillard-Kelly, M.; Yee, D. Detection and downregulation of type I IGF receptor expression by antibody-conjugated quantum dots in breast cancer cells. Breast Cancer Res. Treat. 2009, 114, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Kolter, T.; Sandhoff, K. Principles of Lysosomal Membrane Digestion: Stimulation of Sphingolipid Degradation by Sphingolipid Activator Proteins and Anionic Lysosomal Lipids. Ann. Rev. Cell Develop. Biol. 2005, 21, 81–103. [Google Scholar] [CrossRef]
- Sadik, O.A.; Zhou, A.L.; Kikandi, S.; Du, N.; Wang, Q.; Varner, K. Sensors as tools for quantitation, nanotoxicity and nanomonitoring assessment of engineered nanomaterials. J. Environ. Monit. 2009, 11, 1782–1800. [Google Scholar] [CrossRef] [PubMed]
- Hauck, T.S.; Anderson, R.E.; Fischer, H.C.; Newbigging, S.; Chan, W.C. In vivo quantum-dot toxicity assessment. Small 2010, 6, 138–134. [Google Scholar] [CrossRef] [PubMed]
- Susumu, K.; Uyeda, H.T.; Medintz, I.L.; Mattoussi, H. Design of biotin-functionalized luminescent quantum dots. J. Biomed. Biotechnol. 2007, 7, 90651. [Google Scholar]
- Susumu, K.; Uyeda, H.T.; Medintz, I.L.; Pons, T.; Delehanty, J.B.; Mattoussi, H. Enhancing the stability and biological functionalities of quantum dots via compact multifunctional ligands. J. Am. Chem. Soc. 2007, 129, 13987–13996. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Park, E.; Jo, S.; Song, R. PEG-ylated cationic CdSe/ZnS QDs as an efficient intracellular labeling agent. Phys. Chem. Chem. Phys. 2008, 10, 1739–1742. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Titmuss, S. Polymer-functionalized nanoparticles: from stealth viruses to biocompatible quantum dots. Nanomedicine (London) 2009, 4, 951–966. [Google Scholar] [CrossRef]
- Aillon, K.L.; Xie, Y.; El-Gendy, N.; Berkland, C.J.; Forrest, M.L. Effects of nanomaterial physicochemical properties on in vivo toxicity. Adv. Drug Deliv. Rev. 2009, 61, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Hotz, C.Z. Applications of quantum dots in biology: An overview. Meth. Mol. B. 2005, 303, 1–17. [Google Scholar]
- Haigron, P.; Dillenseger, J.L.; Luo, L.; Coatrieux, J.L. Image-Guided Therapy: Evolution and Breakthrough [A Look At]. IEEE Eng. Med. Biol. Mag. 2010, 29, 100–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saravanakumar, G.; Kim, K.; Park, J.H.; Rhee, K.; Kwon, I.C. Current status of nanoparticle-based imaging agents for early diagnosis of cancer and atherosclerosis. J. Biomed. Nanotechnol. 2009, 5, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Ozpolat, B.; Sood, A.K.; Lopez-Berestein, G. Nanomedicine based approaches for the delivery of siRNA in cancer. J Intern. Med. 2010, 267, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Aiello, M.; Zhou, T.; Berliner, A.; Banerjee, P.; Zhou, S. In situ immobilization of quantum dots in polysaccharide-based nanogels for integration of optical pH-sensing, tumor cell imaging, and drug delivery. Biomaterials 2010, 31, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Orive, G.; Hernández, R.M.; Gascón, A.R.; Pedraz, J.L. Micro and nano drug delivery systems in cancer therapy. Cancer Ther. 2005, 3, 131–138. [Google Scholar]
- Lissett, B.R.; Chang, J.; Fu, K.; Sun, J.; Hu, Y.; Gobin, A.; Yu, T.; Drezek, R.A. Evaluation of Immunotargeted Gold Nanoshells as Rapid Diagnostic Imaging Agents for HER2-Overexpressing Breast Cancer Cells: A Time-based Analysis. Nanobiotechnology 2008, 4, 1–8. [Google Scholar] [CrossRef]
- Bickford, L.R.; Agollah, G.; Drezek, R.; Yu, T.K. Silica-gold nanoshells as potential intraoperative molecular probes for HER2-overexpression in ex vivo breast tissue using near-infrared reflectance confocal microscopy. Breast Cancer Res. Treat. 2009, 120, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, J.; Talavage, T. Gold nanorod/Fe3O4 nanoparticle "nano-pearl-necklaces" for simultaneous targeting, dual-mode imaging, and photothermal ablation of cancer cells. Angew Chem. Int. Ed. Engl. 2009, 48, 2759–2763. [Google Scholar] [CrossRef] [PubMed]
- Kayal, S.; Ramanujan, R.V. Anti-Cancer Drug Loaded Iron-Gold Core-Shell Nanoparticles (Fe@Au) for Magnetic Drug Targeting. J. Nanosci. Nanotechnol. 2010, 10, 1–13. [Google Scholar] [CrossRef]
- Kapoor, Y.; Thomas, J.C.; Tan, G.; John, V.T.; Chauhan, A. Surfactant-laden soft contact lenses for extended delivery of ophthalmic drugs. Biomaterials 2009, 30, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Xinming, L.; Yingde, C.; Lloyd, A.W.; Mikhalovsky, S.V.; Sandeman, S.R.; Howel, C.A.; Liewen, L. Polymeric hydrogels for novel contact lens-based ophthalmic drug delivery systems: A review. Contact Lens & Anterior Eye 2008, 31, 57–64. [Google Scholar] [CrossRef]
- Lü, J.M.; Wang, X.; Marin-Muller, C.; Wang, H.; Lin, P.H.; Yao, Q.; Chen, C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev. Mol. Diagn. 2009, 9, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, A.; Xiong, X.B.; Aliabadi, H.M.; Lavasanifar, A. Polymeric micelles for drug targeting. J. Drug Targeting 2007, 15, 553–584. [Google Scholar] [CrossRef]
- Panda, J.J.; Mishra, A.; Basu, A.; Chauhan, V.S. Stimuli responsive self-assembled hydrogel of a low molecular weight free dipeptide with potential for tunable drug delivery. Biomacromolecules 2008, 9, 2244–2250. [Google Scholar] [CrossRef] [PubMed]
- Van Tomme, S.R.; Storm, G.; Hennink, W.E. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2008, 355, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mincheva, R.; Bougard, F.; Paneva, D.; Vachaudez, M.; Manolova, N.; Rashkov, I.; Dubois, P. Natural polyampholyte-based core-shell nanoparticles with N-carboxyethylchitosan-containing core and poly(ethylene oxide) shell. Biomacromolecules 2009, 10, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Mastrobattista, E.; van der Aa, M.A.E.M.; Hennink, W.E.; Crommelin, D.J.A. Artificial viruses: A nanotechnological approach to gene delivery. Nat. Rev. 2006, 5, 115–121. [Google Scholar]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Mano, J.F. Stimuli-Responsive Polymeric Systems for Biomedical Applications. Adv. Eng. Mat. 2008, 10, 515–527. [Google Scholar] [CrossRef]
- Thierry, B.; Winnik, F.M.; Merhi, Y.; Silver, J.; Tabrizian, M. Bioactive coatings of endovascular stents based on polyelectrolyte multilayers. Biomacromolecules 2003, 4, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.F.; Caruso, F. Facile tailoring of film morphology and release properties using layer-by-layer assembly of thermoresponsive materials. Langmuir 2004, 20, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Decher, G. Fuzzy nanoassemblies: toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Yap, H.P.; Quinn, J.F.; Ng, S.M.; Cho, J.; Caruso, F. Colloid surface engineering via deposition of multilayered thin films from polyelectrolyte blend solutions. Langmuir 2005, 21, 4328–4333. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Kojima, H.; Yamamoto, H.; Toshitada, T.; Hidekazu, T.; Tomaoki, H. Physical stability of size controlled small unilamellar liposomes coated with a modified polyvinyl alcohol. Int. J. Pharm. 1998, 164, 103–111. [Google Scholar]
- Galovic, R.R.; Barisic, K.; Pavelic, Z.; Zanic, G.T.; Cepelak, I.; Filipovic-Grcic, J. High efficiency entrapment of superoxide dismutase into mucoadhesive chitosan-coated liposomes. Eur. J. Pharm. Sci. 2002, 15, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Hillberg, A.L.; Tabrizian, M. Biorecognition through layer-by-layer polyelectrolyte assembly: In-situ hybridization on living cells. Biomacromolecules 2006, 7, 2742–2750. [Google Scholar] [CrossRef] [PubMed]
- Douglas, K.L.; Tabrizian, M. Effect of experimental parameters on the formation of alginate-chitosan nanoparticles and evaluation of their potential application as DNA carrier. J. Biomater. Sci. Polym. Ed. 2005, 16, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Gref, R.; Minamitake, Y.; Perracchia, M.T.; Trubeskoy, V.; Torchilin, V.; Langer, R. Biodegradable long-circulating polymeric nanospheres. Science 1994, 263, 1600–1603. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.P.; Labhasetwar, V.; Amidon, G.L.; Levy, R.J. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm. Res. 1996, 13, 1838–1845. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Valentini, R.F. Retention and activity of BMP-2 in hyaluronic acid-based scaffolds in vitro. J. Biomed. Mater. Res. 2002, 59, 573–584. [Google Scholar] [PubMed]
- Sydow-Plum, G.; Haidar, Z.S.; Merhi, Y.; Tabrizian, M. Modulating the Release Kinetics of Paclitaxel from Membrane-Covered Stents Using Different Loading Strategies. Materials 2008, 1, 25–43. [Google Scholar] [CrossRef]
- Douglas, K.L.; Piccirillo, C.A.; Tabrizian, M. Effects of alginate inclusion on the vector properties of chitosan-based nanoparticles. J. Control. Release 2006, 27, 354–361. [Google Scholar] [CrossRef]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; McMillan, N.A. Lipidic systems for in vivo siRNA delivery. AAPS J. 2009, 11, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Franzen, S.; Lommel, S.A. Targeting cancer with 'smart bombs': equipping plant virus nanoparticles for a 'seek and destroy' mission. Nanomed (London) 2009, 4, 575–588. [Google Scholar] [CrossRef]
- Chan, J.M.; Zhang, L.; Yuet, K.P.; Liao, G.; Rhee, J.W.; Langer, R.; Farokhzad, O.C. PLGA-lecithin-PEG core-shell nanoparticles for controlled drug delivery. Biomaterials 2009, 30, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Kenley, R.; Marden, L.; Turek, T.; Jin, L.; Ron, E.; Hollinger, J.O. Osseous regeneration in the rat calvarium using novel delivery systems for recombinant human bone morphogenetic protein-2 (rhBMP-2). J. Biomed. Mater. Res. 1994, 28, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Kinoshita, A.; Takahashi, K.; Oda, S.; Ishikawa, I. Bone regeneration by recombinant human bone morphogenetic protein-2 in rat mandibular defects. An experimental model of defect filling. J. Periodontol. 1999, 70, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Jin, Q.; Giannobile, W.V.; Ma, P.X. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials 2007, 28, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Corsi, F.; De Palma, C.; Colombo, M.; Allevi, R.; Nebuloni, M.; Ronchi, S.; Rizzi, G.; Tosoni, A.; Trabucchi, E.; Clementi, E.; Prosperi, D. Towards ideal magnetofluorescent nanoparticles for bimodal detection of breast-cancer cells. Small 2009, 5, 2555–2564. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.I.; Kim, J.C.; Kim, Y.H.; Tae, G.; Lee, S.Y.; Kim, K.; Kwon, I.C. The effect of surface functionalization of PLGA nanoparticles by heparin- or chitosan-conjugated Pluronic on tumor targeting. J. Control. Release 2010, 143, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Adiseshaiah, P.P.; Hall, J.B.; McNeil, S.E. Nanomaterial standards for efficacy and toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Osada, K.; Christie, J.R.; Kataoka, K. Polymeric micelles from poly(ethylene glycol)-poly(amino acid) block copolymer for drug and gene delivery. J. R. Soc. Interface 2009, 6, S325–S339. [Google Scholar] [PubMed]
- Ma, P.X. Biomimetic Materials for Tissue Engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Gobin, A.M.; Lee, M.H.; Halas, N.J.; James, W.D.; Drezek, R.A.; West, J.L. Near-Infrared Resonant Nanoshells for Combined Optical Imaging and Photothermal Cancer Therapy. Nano Lett. 2007, 7, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Ahn, J.S.; Lim, J.I.; Lee, Y.K. Influence of TiO2 nanoparticles on the optical properties of resin composites. Dent. Mater. 2009, 25, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Ramaswamy, N. Electrochemical surface modification of titanium in dentistry. Dent. Mater. 2009, 28, 20–36. [Google Scholar] [CrossRef]
- Xiaa, Y.; Zhanga, F.; Xiea, H.; Gub, N. Nanoparticle-reinforced resin-based dental composites. J. Dentistry 2008, 36, 450–455. [Google Scholar] [CrossRef]
- Alves, L.P.; Pilla, V.; Murgo, D.O.A.; Munin, E. Core-shell quantum dots tailor the fluorescence of dental resin composites. J. Dentistry 2010, 38, 149–152. [Google Scholar] [CrossRef]
- Moszner, N.; Klapdohr, S. Nanotechnology for dental composites. Int. J. Nanotechnol. 2004, 1, 130–156. [Google Scholar]
- De la Isla, A.; Brostow, W.; Bujard, B.; Estevez, M.; Rodriguez, J.R.; Vargas, S.; Castano, V.M. Nanohybrid scratch resistant coating for teeth and bone viscoelasticity manifested in tribology. Mater. Res. Innov. 2003, 7, 110–114. [Google Scholar]
- Berube, D.M. The magic of nano. Nanotechnol. Percept. 2006, 2, 249–255. [Google Scholar]
- Ballauff, M.; Lu, Y. "Smart" Nanoparticles: Preparation, Characterization and Applications. Polymer 2007, 48, 1815–1823. [Google Scholar] [CrossRef]
- Welsch, N.; Wittemann, A.; Ballauff, M. Enhanced activity of enzymes immobilized in thermoresponsive core-shell microgels. J. Phys. Chem. B 2009, 113, 16039–16045. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.P.; Sharma, U.; Mikos, G. A. Electrospinning of Polymeric Nanofibers for Tissue Engineering Applications: A Review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [PubMed]
- Pillai, C.K.S.; Sharma, C. P. Electrospinning of Chitin and Chitosan Nanofibres. Trends Biomater. Artif. Organ. 2009, 22, 179–201. [Google Scholar]
- Liao, I.C.; Chew, S.Y.; Leong, K.W. Aligned core-shell nanofibers delivering bioactive proteins. Nanomedicine 2006, 1, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Katti, D.S.; Robinson, K.W.; Ko, F.K.; Laurencin, C.T. Bioresorbable nanofiber-based systems for wound healing and drug delivery: optimization of fabrication parameters. Biomed. Mater. Res. B Appl. Biomater. 2004, 70, 286–296. [Google Scholar] [CrossRef]
- Tian, X.; Fei, J.; Pi, Z.; Yang, C.; Luo, D. Synthesis and characterization of amoxicillin nanostructures. Nanomedicine 2005, 1, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, M.; Markova, N.; Manolova, N.; Rashkov, I. Antibacterial and antimycotic activity of a cross-linked electrospun poly(vinyl pyrrolidone)-iodine complex and a poly(ethylene oxide)/poly(vinyl pyrrolidone)-iodine complex. J. Biomater. Sci. Polym. Ed. 2008, 19, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, X.; Liu, Y.; Su, Q.; Qiang, M.L.; Mo, X. Encapsulation and Controlled Release of Heparin from Electrospun Poly(L-Lactide-co-epsilon-Caprolactone) Nanofibers. J. Biomater. Sci. Polym. Ed. 2010. in print. [Google Scholar]
- Dubois, G.; Segers, V.F.; Bellamy, V.; Sabbah, L.; Peyrard, S.; Bruneval, P.; Hagège, A.A.; Lee, R.T.; Menasché, P. Self-assembling peptide nanofibers and skeletal myoblast transplantation in infarcted myocardium. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.C.; Davis, M.E.; Gannon, J.; MacGillivray, C.; Lee, R.T. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J. Clin. Invest. 2006, 116, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, H.; Hosseinkhani, M.; Khademhosseini, A.; Kobayashi, H. Bone regeneration through controlled release of bone morphogenetic protein-2 from 3-D tissue engineered nano-scaffold. J. Control. Release 2007, 117, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Kaji, N.; Okamoto, Y.; Tokeshi, M.; Baba, Y. Nanopillar, nanoball, and nanofibers for highly efficient analysis of biomolecules. Chem. Soc. Rev. 2010, 39, 948–956. [Google Scholar] [CrossRef] [PubMed]
- McKnight, T.E.; Melechko, A.V.; Guillorn, M.A.; Merkulov, V.I.; Lowndes, D.H.; Simpson, M.L. Synthetic nanoscale elements for delivery of materials into viable cells. Methods Mol. B. 2005, 303, 191–208. [Google Scholar]
- Choi, S.W.; Park, J.Y.; Kim, S.S. Synthesis of SnO2–ZnO core–shell nanofibers via a novel two-step process and their gas sensing properties. Nanotechnology 2009, 20, 465603. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Haidar, Z.S. Bio-Inspired/-Functional Colloidal Core-Shell Polymeric-Based NanoSystems: Technology Promise in Tissue Engineering, Bioimaging and NanoMedicine. Polymers 2010, 2, 323-352. https://doi.org/10.3390/polym2030323
Haidar ZS. Bio-Inspired/-Functional Colloidal Core-Shell Polymeric-Based NanoSystems: Technology Promise in Tissue Engineering, Bioimaging and NanoMedicine. Polymers. 2010; 2(3):323-352. https://doi.org/10.3390/polym2030323
Chicago/Turabian StyleHaidar, Ziyad S. 2010. "Bio-Inspired/-Functional Colloidal Core-Shell Polymeric-Based NanoSystems: Technology Promise in Tissue Engineering, Bioimaging and NanoMedicine" Polymers 2, no. 3: 323-352. https://doi.org/10.3390/polym2030323
APA StyleHaidar, Z. S. (2010). Bio-Inspired/-Functional Colloidal Core-Shell Polymeric-Based NanoSystems: Technology Promise in Tissue Engineering, Bioimaging and NanoMedicine. Polymers, 2(3), 323-352. https://doi.org/10.3390/polym2030323