Miscibility and Hydrogen Bonding in Blends of Poly(4-vinylphenol)/Poly(vinyl methyl ketone)
Abstract
:1. Introduction
2. Experimental Section
| Polymers | Mw | Mw/Mn | Tg (°C) |
|---|---|---|---|
| PVPh | 150,000 | 1.74 | 158 |
| PVMK | 500,000 | – | 37.5 |
3. Results and Discussion
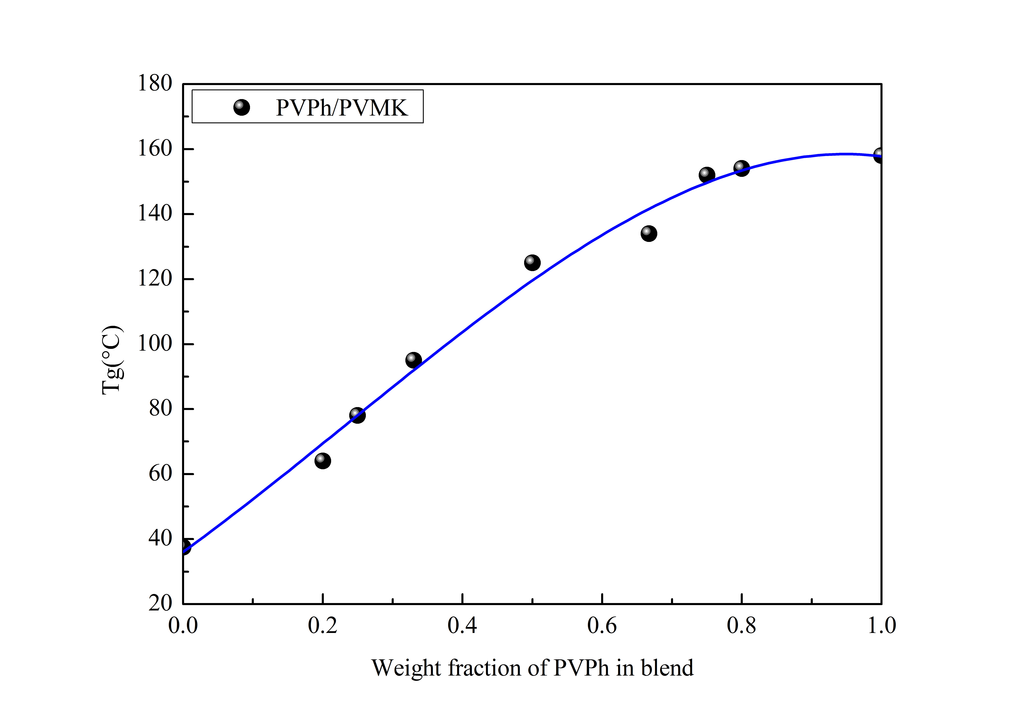
3.1. DSC Study



3.2. FTIR Study





| Blend Composition (PVMK/PVPh) | Free C=O | Hydrogen Bonded C=O | ||||
|---|---|---|---|---|---|---|
| Frequency ν (cm−1) | Width w1/2 (cm−1) | Fraction ffree | Frequency ν (cm−1) | Width w1/2 (cm−1) | Fraction fAsso | |
| 80/20 | 1710 | 20 | 0.770 | 1705 | 19 | 0.232 |
| 75/25 | 1710 | 21 | 0.681 | 1704 | 18 | 0.319 |
| 67/33 | 1710 | 21 | 0.588 | 1705 | 19 | 0.412 |
| 50/50 | 1709 | 21 | 0.467 | 1704 | 19 | 0.533 |
| 33/67 | 1710 | 20 | 0.369 | 1705 | 20 | 0.631 |
| 25/75 | 1710 | 22 | 0.326 | 1706 | 19 | 0.674 |
| 20/80 | 1709 | 21 | 0.307 | 1704 | 18 | 0.693 |

3.3. SEM Study

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Utracki, L.A. Polymer Blends Handbook; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; Volume 2. [Google Scholar]
- Paul, D.R.; Newman, S. Polymer Blends; Academic Press: New York, NY, USA, 1978; Volume 1. [Google Scholar]
- Coleman, M.M.; Graf, J.F.; Painter, P.C. Specific Interactions and the Miscibility of Polymer Blends; Technomic Publishing: Lancaster, PA, USA, 1991. [Google Scholar]
- Motzer, H.R.; Painter, P.C.; Coleman, M.M. Interactions in miscible in miscible blends of poly(styrene-co-methacrylic acid) with copolymers containing vinylpyrrolidone and vinylpyridine groups. Macromolecules 2001, 34, 8390–8393. [Google Scholar]
- Lee, J.Y.; Painter, P.C.; Coleman, M.M. Hydrogen bonding in polymer blends. 3. Blends involving polymers containing methacrylic acid and ethergroups. Macromolecules 1988, 21, 346–354. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiang, M.; Jiang, M.; Wu, C. Complexation between poly(styrene-co-4-vinylphenol) and poly(styrene-co-4-vinylpyridine) in solution. Macromolecules 1997, 30, 6084–6089. [Google Scholar] [CrossRef]
- Xiang, M.; Jiang, M.; Zhang, Y.; Wu, C.; Feng, L. Intermacromolecular complexation due to specific interactions. 4. The hydrogen-bonding complex of vinylphenol-containing copolymer and vinylpyridine containing copolymer. Macromolecules 1997, 30, 2313–2319. [Google Scholar] [CrossRef]
- Prinos, A.; Dompros, A.; Panayiotou, C. Thermoanalytical and spectroscopic study of poly(vinylpyrrolidone)/poly(styrene-co-vinylphenol) blends. Eur. Polym. J. 1998, 39, 3011–3016. [Google Scholar] [CrossRef]
- Benabdelghani, Z.; Etxeberria, A. The phase behavior and thermal stability of blends of poly(styrene-co-methacrylic acid)/poly(styrene-co-4-vinylpyridine). J. Appl. Polym. Sci. 2011, 121, 462–468. [Google Scholar] [CrossRef]
- De Meftahi, M.V.; Frechet, J.M.J. Study of the compatibility of blends of polymers and copolymers containing styrene, 4-hydroxystyrene and 4-vinylpyridine. Polymer 1988, 29, 477–482. [Google Scholar] [CrossRef]
- Li, D.; Brisson, J. DMTA and FTIR investigation of the phase behavior of poly(methyl methacrylate)–poly(4-vinylphenol) blends. Macromolecules 1996, 29, 868–874. [Google Scholar] [CrossRef]
- Painter, P.C.; Graf, J.F.; Coleman, M.M. Effect of hydrogen bonding on the enthalpy of mixing and the composition dependence of the glass transition temperature in polymer. Macromolecules 1991, 24, 5630–5638. [Google Scholar] [CrossRef]
- Yi, J.; Goh, S.H.; Wee, A.T.S. Miscibility and interactions in poly(methylthiomethyl methacrylate)/poly(p-vinylphenol) blends. Macromolecules 2001, 34, 7411–7415. [Google Scholar] [CrossRef]
- Belabed, Ch.; Benabdelghani, Z.; Granado, A.; Etxeberria, A. Miscibility and specific interactions in blends of poly(4-vinylphenol-co-methyl methacrylate)/poly(styrene-co-4-vinylpyridine). J. Appl. Polym. Sci. 2012, 125, 3811–3819. [Google Scholar] [CrossRef]
- Kuo, S.W.; Chang, F.C. Studies of miscibility behavior and hydrogen bonding in blends of poly(vinylphenol) and poly(vinylpyrrolidone). Macromolecules 2001, 34, 5224–5228. [Google Scholar] [CrossRef]
- Qin, C.; Pires, A.T.N.; Belfiore, L.A. Spectroscopic investigations of specific interactions in amorphous polymer–polymer blends: Poly(vinylphenol) and poly(vinyl methyl ketone). Macromlecules 1991, 24, 666–670. [Google Scholar] [CrossRef]
- Lee, L.T.; Woo, E.M.; Hou, S.S.; Förster, S. Miscibility with positive deviation in Tg-composition relationship in blends of poly(2-vinyl pyridine)-block-poly(ethylene oxide) and poly(p-vinyl phenol). Polymer 2006, 47, 8350–8359. [Google Scholar]
- Wang, J.; Cheung, M.K.; Mi, Y. Miscibility in blends of poly (4-vinylpyridine)/poly (4-vinylphenol) as studied by 13C solid-state NMR. Polymer 2001, 42, 3087–3093. [Google Scholar] [CrossRef]
- Vogel, C.; Wessel, E.; Siesler, H.W. FTIR spectroscopic imaging of anisotropic poly(3-hydroxybutyrate)/poly(lactic acid) blends with polarized radiation. Macromolecules 2008, 41, 2975–2977. [Google Scholar] [CrossRef]
- Guo, Q.; Huang, J.; Li, X. Miscibility of poly(vinyl methyl ketone) with poly(2-hydroxyethyl methacrylate) and poly(epichlorohydrin). Eur. Polym. J. 1996, 3, 321–323. [Google Scholar] [CrossRef]
- Yi, J.Z.; Goh, S.H. Miscibility and interactions in poly(methylthiomethyl methacrylate)/poly(vinyl alcohol) blends. Polymer 2003, 44, 1973–1978. [Google Scholar] [CrossRef]
- Hill, D.J.T.; Whittaker, A.K.; Wong, K.W. Miscibility and specific interactions in blends of poly(4-vinylphenol) and poly(2-ethoxyethyl methacrylate). Macromolecules 1999, 32, 5285–5291. [Google Scholar] [CrossRef]
- Kuo, S.W.; Chang, F.C. Miscibility and hydrogen bonding in blends of poly(vinylphenol-co-methyl methacrylate) with poly(ethylene oxide). Macromolecules 2001, 34, 4089–4097. [Google Scholar] [CrossRef]
- Wu, Y.S.; Wu, Y.C.; Kuo, S.W. Thymine and adenine-functionalized polystyrene form self-assembled structures through multiple complementary hydrogen bonds. Polymers 2014, 6, 1827–1845. [Google Scholar] [CrossRef]
- Zhu, B.; He, Y.; Yoshie, N.; Asakawa, N.; Inoue, Y. Partial phase segregation in strongly hydrogen-bonded and miscible blends. Macromolecules 2004, 37, 3257–3266. [Google Scholar] [CrossRef]
- Benabdelghani, Z.; Etxeberria, A. Hydrogen bonds in blends of poly(vinylphenol-co-methyl methacrylate)/poly(vinyl methyl ketone). Macromol. Symp. 2012, 321–322, 170–174. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourara, H.; Hadjout, S.; Benabdelghani, Z.; Etxeberria, A. Miscibility and Hydrogen Bonding in Blends of Poly(4-vinylphenol)/Poly(vinyl methyl ketone). Polymers 2014, 6, 2752-2763. https://doi.org/10.3390/polym6112752
Bourara H, Hadjout S, Benabdelghani Z, Etxeberria A. Miscibility and Hydrogen Bonding in Blends of Poly(4-vinylphenol)/Poly(vinyl methyl ketone). Polymers. 2014; 6(11):2752-2763. https://doi.org/10.3390/polym6112752
Chicago/Turabian StyleBourara, Hana, Slimane Hadjout, Zitouni Benabdelghani, and Agustin Etxeberria. 2014. "Miscibility and Hydrogen Bonding in Blends of Poly(4-vinylphenol)/Poly(vinyl methyl ketone)" Polymers 6, no. 11: 2752-2763. https://doi.org/10.3390/polym6112752
APA StyleBourara, H., Hadjout, S., Benabdelghani, Z., & Etxeberria, A. (2014). Miscibility and Hydrogen Bonding in Blends of Poly(4-vinylphenol)/Poly(vinyl methyl ketone). Polymers, 6(11), 2752-2763. https://doi.org/10.3390/polym6112752