Casein Films: The Effects of Formulation, Environmental Conditions and the Addition of Citric Pectin on the Structure and Mechanical Properties
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Film-Making Solutions
| Formulation | Components 1 + 2 | Component 3 | Component 4 |
|---|---|---|---|
| Control A | Water + CaCas | Gly | - |
| A | Water + CaCas | Gly | CPsol |
| B | Water + CaCas | CPsol | Gly |
| E | Water + Gly | CaCas | CPsol |
| Control F | Water + Gly | CaCas | - |
| F | Water + Gly | CPsol | CaCas |
| G | Water + CPsol | CaCas | Gly |
| H | Water + CPsol | Gly | CaCas |
| K | CPsol + Gly | Water | CaCas |
| Component | Molecular Weight | Solid Fraction | Mole Fraction | Binding Potential |
|---|---|---|---|---|
| Caseins | 19,000–25,000 g/mol | ~73 wt% | ~1.2% | Multiple functional groups, including –NH, and (+) and (−) charges. Assembly into micelles. |
| Glycerol | 92 g/mol | ~25 wt% | ~89.7% | Small, polar, 3 –OH groups |
| Calcium ions | 40 g/mol | ~1.1 wt% | ~9.1% | Ca2+, 2 positive charges |
| Citric Pectin | 236,000 g/mol | 0 to 1 wt% | 0 to 0.0014% | Long, linear, –OH and –COO− groups |
2.3. Film-Casting Procedure
2.4. Tensile Properties Measurement
2.5. Microscopy Imaging
3. Results and Discussion
3.1. Control A and F Films: The Effects of Humidity and Thickness on Tensile Properties


3.2. Incorporation of Citric Pectin (CP) to Calcium Caseinate Films
- Strong binding of –COO− from two pectin molecules with Ca2+, forming a pectin gel,
- Ionic binding of Ca2+ with two negatively-charged groups on caseins,
- Electrostatic binding of Ca2+ with several –OH groups,
- Binding of –OH groups on glycerol with positively-charged groups on caseins,
- Binding of –OH and –COO− groups on pectin chains with positively-charged casein groups, such as –NH [44],
- Dipole-charge and/or dipole-dipole interactions [44],
- Other,
- Any combination of the above.
| CP Content | Modulus, E | Strength, S | Elongation, EAB |
|---|---|---|---|
| 0% (Control F) | 6 MPa | 16 Mpa | 20% |
| 0.05% | 10 Mpa | 26 Mpa | 12% |
| 0.4% | 13 Mpa | 33 Mpa | 7% |
| 0.95% | 8 Mpa | 22 Mpa | 3% |

3.3. Micrographs of the Films at Different CP Contents


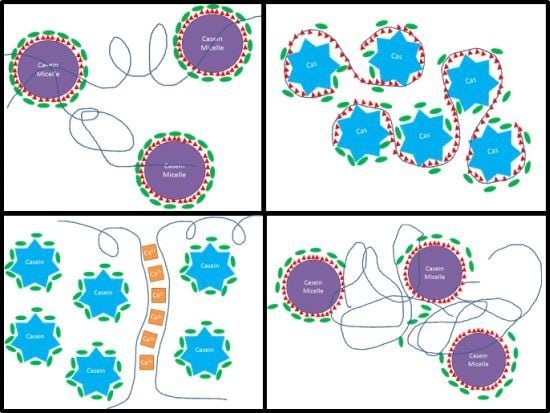
3.4. Mechanistic Interpretation
| Form | Mixing Sequence | Hypothetic Mechanism | Possible Network Structure | Schematic |
|---|---|---|---|---|
| A | 1. Water + CaCas 2. Gly 3. CPsol | 1. Casein micelles + calcium ions. 2. Glycerol coats casein micelles. 3. Coiled pectin loosely binds to a few available micelles sites and strings micelles together, while excess pectin forms gels with calcium. | 0.3% CP: coiled pectin acts as an elastic chain-extender for the micelles network (“pearl necklace” structure), increasing EAB and reducing S and E. 1% CP: random, isolated pockets of CP/Ca2+ gel become larger. Steric disruption of micelles network increases S and E, greatly reduces EAB. |  |
| B | 1. Water + CaCas 2. CPsol 3. Gly | 1. Casein micelles network + calcium ions. 2. Some coiled pectins interconnect casein micelles using the many available sites, while others form gels with calcium. 3. Glycerol coats the whole casein/pectin network. | 0.3% CP: similar to A, but stiffer and less stretchy, because coiled pectins bind more strongly to uncoated micelles. 1% CP: additional pectin chains both reinforce the micelles network and form more gel pockets, increasing E and S and moderately reducing mobility and EAB. |  |
| E | 1. Water + Gly 2. CaCas 3. CPsol | 1. Casein molecules and/or micelles become fully coated with glycerol during mixing; calcium ions in solution. 2. Coiled pectin intercalates between caseins, with few available casein sites to bind with; at a higher CP concentration, pectin chains find each other and gel with calcium. | 0.3% CP: coiled pectin disperses homogeneously and acts as chain-extender for casein network, increasing EAB; steric disruption of network reduces S and E. 1% CP: narrow, interconnected pockets of hard CP/Ca2+ gel stiffen the network like bones and prevent mobility and elongation. |  |
| F | 1. Water + Gly 2. CPsol 3. CaCas | 1. Pectin chains become fully hydrated (uncoiled) and coated with glycerol. 2. Casein molecules attach to a few available sites on the pectin chains. Short pectin sections may gel together with calcium ions. 3. Excess caseins form micelle network. | 0.3% CP: network of casein micelles, with segregated pockets of pectin grafted with caseins and crosslinked to other pectin chains by short gel sections; pectin chains provide mobility, increase elongation and reduce stiffness. 1% CP: higher pectin:casein ratio produces a homogeneous network of casein-grafted pectins; denser gel-crosslinks increase stiffness and reduce mobility and elongation. |  |
| G | 1. Water + CPsol 2. CaCas 3. Gly | 1. Fully hydrated, uncoiled pectin chains. 2. Pectin wraps around casein molecules tightly. Excess caseins form micelle network. 3. Glycerol coats pectin/casein complexes and micelles. | 0.3% CP: network of casein micelles, with small segregated pockets of pectin/casein complexes that increase strength of the micelle network while slightly hindering mobility. 1% CP: higher pectin:casein ratio produces a homogeneous network of pectin/casein complexes; less caseins per pectin chain form a looser network with increased mobility, lower stiffness. |  |
| H | 1. Water + CPsol 2. Gly 3. CaCas | 1. Fully hydrated, uncoiled pectin chains, slightly coated with glycerol. 2. With fewer available sites, pectin wraps loosely around caseins. Excess caseins form micelle network. 3. Unbound pectin chain sections gel together with calcium ions. | 0.3% CP: Similar to G; however, looser pectin/casein complexes with more gelled pectin chain sections form larger, looser segregated pockets in the micelle network and affect E, S and EAB less. 1% CP: higher pectin:casein ratio produces a homogeneous network of loose pectin/casein complexes; less caseins per pectin chain form a looser network with increased mobility, lower stiffness. |  |

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Han, J.H.; Gennadios, A. Edible films and coatings: A review. In Innovations in Food Packaging; Han, J.H., Ed.; Elsevier: San Diego, CA, USA, 2005; pp. 239–262. [Google Scholar]
- Elzoghby, A.O.; El-Fotoh, W.S.; Elgindy, N.A. Casein-based formulations as promising controlled release drug delivery systems. J. Controll. Release 2011, 153, 206–216. [Google Scholar] [CrossRef]
- Gennadios, A. Edible films and coatings from proteins. In Proteins in Food Processing; Yada, R.Y., Ed.; Woodhead Publishing: Cambridge, UK, 2004; pp. 442–467. [Google Scholar]
- Miller, K.S.; Krochta, J.M. Oxygen and aroma barrier properties of edible films: A review. Trends Food Sci. Technol. 1997, 8, 228–237. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peltzer, M.A.; López, J.; Garrigós, M.D.C.; Valente, A.J.M.; Jiménez, A. Functional properties of sodium and calcium caseinate antimicrobial active films containing carvacrol. J. Food Eng. 2014, 121, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, E. Surface treatments and edible coatings in food preservation. In Handbook of Food Preservation, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 477–507. [Google Scholar]
- Ginger, M.R.; Grigor, M.R. Comparative aspects of milk caseins. Comp. Biochem. Physiol. Part B 1999, 125, 133–145. [Google Scholar]
- Dangaran, K.L.; Tomasula, P.M.; Qi, P. Structure and function of protein-based edible films and coatings. In Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; pp. 25–56. [Google Scholar]
- Kalicka, D.; Najgebauer-Lejko, D.; Grega, T. Nonfood applications of milk proteins: A review. In Colloids in Biotechnology; Fanun, M., Ed.; CRC Press: Boca Raton, FL, USA, 2010; Volume 152; pp. 151–175. [Google Scholar]
- Tomasula, P.M.; Yee, W.C.; Parris, N. Oxygen permeability of films made from CO2-precipitated casein and modified casein. J. Agric. Food Chem. 2003, 51, 634–639. [Google Scholar] [CrossRef]
- Abu Diak, O.; Bani-Jaber, A.; Amro, B.; Jones, D.; Andrews, G.P. The manufacture and characterization of casein films as novel tablet coatings. Food Bioprod. Proc. 2007, 85, 284–290. [Google Scholar] [CrossRef]
- Longares, A.; Monahan, F.J.; O’Riordan, E.D.; O’Sullivan, M. Physical properties of edible films made from mixtures of sodium caseinate and wpi. Int. Dairy J. 2005, 15, 1255–1260. [Google Scholar] [CrossRef]
- Ghosh, A.; Ali, M.A.; Dias, G.J. Effect of cross-linking on microstructure and physical performance of casein protein. Biomacromolecules 2009, 10, 1681–1688. [Google Scholar] [CrossRef]
- Dangaran, K.L.; Cooke, P.; Tomasula, P.M. The effect of protein particle size reduction on the physical properties of CO2-precipitated casein films. J. Food Sci. 2006, 71, E196–E201. [Google Scholar] [CrossRef]
- Mauer, L.J.; Smith, D.E.; Labuza, T.P. Water vapor permeability, mechanical, and structural properties of edible beta-casein films. Int. Dairy J. 2000, 10, 353–358. [Google Scholar] [CrossRef]
- Tomasula, P.M. Using dairy ingredients to produce edible films and biodegradable packaging materials. In Dairy-Derived Ingredients: Food and Nutraceutical Uses; Corredig, M., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 589–624. [Google Scholar]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Chick, J.; Ustunol, Z. Mechanical and barrier properties of lactic acid and rennet precipitated casein-based edible films. J. Food Sci. 1998, 63, 1024–1027. [Google Scholar]
- Tomasula, P.M. Edible, Water-Solubility Resistant Casein Masses. US 6,379,726 B1, 30 April 2002. [Google Scholar]
- Kozempel, M.; Tomasula, P.M. Development of a semi-continuous process for CO2-precipitated-casein films. Abstr. Pap. Am. Chem. Soc. 2005, 229, U302. [Google Scholar]
- Chambi, H.; Grosso, C. Edible films produced with gelatin and casein cross-linked with transglutaminase. Food Res. Int. 2006, 39, 458–466. [Google Scholar] [CrossRef]
- Wihodo, M.; Moraru, C.I. Physical and chemical methods used to enhance the structure and mechanical properties of protein films: A review. J. Food Eng. 2013, 114, 292–302. [Google Scholar]
- Ouattara, B.; Canh, L.T.; Vachon, C.; Mateescu, M.A.; Lacroix, M. Use of gamma-irradiation cross-linking to improve the water vapor permeability and the chemical stability of milk protein films. Radiat. Phys. Chem. 2002, 63, 821–825. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Albuquerque, R.L.C.; Padilha, F.F.; Bittencourt, F.O.; de Freitas, O.; Nunes, P.S.; Pereira, N.L.; Fonseca, M.J.V.; Araujo, A.A.S. Effect of the maillard reaction on properties of casein and casein films. J. Therm. Anal. Calorim. 2011, 104, 249–254. [Google Scholar] [CrossRef]
- Rezvani, E.; Schleining, G.; Sümen, G.; Taherian, A.R. Assessment of physical and mechanical properties of sodium caseinate and stearic acid based film-forming emulsions and edible films. J. Food Eng. 2013, 116, 598–605. [Google Scholar] [CrossRef]
- Juvonen, H.; Smolander, M.; Boer, H.; Pere, J.; Buchert, J.; Peltonen, J. Film formation and surface properties of enzymatically crosslinked casein films. J. Appl. Polym. Sci. 2011, 119, 2205–2213. [Google Scholar] [CrossRef]
- Li, X.; Fang, Y.; Phillips, G.O.; Al-Assaf, S. Improved sugar beet pectin-stabilized emulsions through complexation with sodium caseinate. J. Agric. Food Chem. 2013, 61, 1388–1396. [Google Scholar] [CrossRef]
- Baracat, M.M.; Nakagawa, A.M.; Casagrande, R.; Georgetti, S.R.; Verri, W.A.; de Freitas, O. Preparation and characterization of microcapsules based on biodegradable polymers: Pectin/casein complex for controlled drug release systems. AAPS PharmSciTech 2012, 13, 364–372. [Google Scholar]
- Cucheval, A.; Al-Ghobashy, M.A.; Hemar, Y.; Otter, D.; Williams, M.A.K. Direct measurements of interfacial interactions between pectin and k-casein and implications for the stabilisation of calcium-free casein micelle mimics. J. Colloid Interface Sci. 2009, 338, 450–462. [Google Scholar] [CrossRef]
- Tuinier, R.; Rolin, C.; de Kruif, C.G. Electrosorption of pectin onto casein micelles. Biomacromolecules 2002, 3, 632–638. [Google Scholar] [CrossRef]
- Maroziene, A.; de Kruif, C.G. Interaction of pectin and casein micelles. Food Hydrocoll. 2000, 14, 391–394. [Google Scholar] [CrossRef]
- Pedersen, H.C.A.; Jorgensen, B.B. Influence of pectin on the stability of casein solutions studied in dependence of varying pH and salt concentration. Food Hydrocoll. 1991, 5, 323–328. [Google Scholar] [CrossRef]
- Kurita, O.; Fujiwara, T.; Yamazaki, E. Characterization of the pectin extracted from citrus peel in the presence of citric acid. Carbohydr. Polym. 2008, 74, 725–730. [Google Scholar] [CrossRef]
- Thakur, B.R.; Singh, R.K.; Handa, A.K.; Rao, M.A. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef]
- Brejnholt, S.M. Pectin. In Food Stabilisers, Thickeners and Gelling Agents; Imeson, A., Ed.; John Wiley & Sons: New York, NY, USA, 2010; pp. 237–265. [Google Scholar]
- Löfgren, C.; Hermansson, A.-M. Synergistic rheological behaviour of mixed hm/lm pectin gels. Food Hydrocoll. 2007, 21, 480–486. [Google Scholar]
- Sejersen, M.T.; Salomonsen, T.; Ipsen, R.; Clark, R.; Rolin, C.; Engelsen, S.B. Zeta potential of pectin-stabilised casein aggregates in acidified milk drinks. Int. Dairy J. 2007, 17, 302–307. [Google Scholar]
- Liu, J.R.; Nakamura, A.; Corredig, M. Addition of pectin and soy soluble polysaccharide affects the particle size distribution of casein suspensions prepared from acidified skim milk. J. Agric. Food Chem. 2006, 54, 6241–6246. [Google Scholar] [CrossRef]
- Letendre, M.; D’Aprano, G.; Lacroix, M.; Salmieri, S.; St-Gelais, D. Physicochemical properties and bacterial resistance of biodegradable milk protein films containing agar and pectin. J. Agric. Food Chem. 2002, 50, 6017–6022. [Google Scholar] [CrossRef]
- Somanathan, N. Effect of environmental factors on the mechanical properties of grafted casein films: Influence of humidity and biaxial orientation. J. Appl. Polym. Sci. 1996, 62, 1407–1414. [Google Scholar] [CrossRef]
- Cuq, B.; Gontard, N.; Aymard, C.; Guilbert, S. Relative humidity and temperature effects on mechanical and water vapor barrier properties of myofibrillar protein-based films. Polym. Gels Netw. 1997, 5, 1–15. [Google Scholar] [CrossRef]
- Gennadios, A.; Park, H.J.; Weller, C.L. Relative-humidity and temperature effects on tensile-strength of edible protein and cellulose ether films. T ASAE 1993, 36, 1867–1872. [Google Scholar]
- Tomasula, P.M.; Parris, N.; Yee, W.; Coffin, D. Properties of films made from CO2-precipitated casein. J. Agric. Food Chem. 1998, 46, 4470–4474. [Google Scholar] [CrossRef]
- Zaleska, H.; Mazurkiewicz, J.; Tomasik, P.; Baczkowicz, M. Electrochemical synthesis of polysaccharide-protein complexes. Part 2. Apple pectin-casein complexes. Nahrung 1999, 43, 278–283. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bonnaillie, L.M.; Zhang, H.; Akkurt, S.; Yam, K.L.; Tomasula, P.M. Casein Films: The Effects of Formulation, Environmental Conditions and the Addition of Citric Pectin on the Structure and Mechanical Properties. Polymers 2014, 6, 2018-2036. https://doi.org/10.3390/polym6072018
Bonnaillie LM, Zhang H, Akkurt S, Yam KL, Tomasula PM. Casein Films: The Effects of Formulation, Environmental Conditions and the Addition of Citric Pectin on the Structure and Mechanical Properties. Polymers. 2014; 6(7):2018-2036. https://doi.org/10.3390/polym6072018
Chicago/Turabian StyleBonnaillie, Laetitia M., Han Zhang, Serife Akkurt, Kit L. Yam, and Peggy M. Tomasula. 2014. "Casein Films: The Effects of Formulation, Environmental Conditions and the Addition of Citric Pectin on the Structure and Mechanical Properties" Polymers 6, no. 7: 2018-2036. https://doi.org/10.3390/polym6072018
APA StyleBonnaillie, L. M., Zhang, H., Akkurt, S., Yam, K. L., & Tomasula, P. M. (2014). Casein Films: The Effects of Formulation, Environmental Conditions and the Addition of Citric Pectin on the Structure and Mechanical Properties. Polymers, 6(7), 2018-2036. https://doi.org/10.3390/polym6072018