Multi-Channeled Polymeric Microsystem for Studying the Impact of Surface Topography on Cell Adhesion and Motility
Abstract
:1. Introduction
2. Experimental Section
2.1. Design Process
2.2. Manufacturing Process

| Channel number | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Alpha (α) | 0.9 | 0.7 | 0.5 | 0.3 | 0.1 |
| Fractal dimension | 2.1 | 2.3 | 2.5 | 2.7 | 2.9 |
| Surface roughness (Ra), microns | 24.3 | 27.8 | 33.2 | 37.8 | 40.9 |
| Surface roughness (Rrms), microns | 31.6 | 35.2 | 42.6 | 50.5 | 53.6 |
| Surface roughness (Rt), microns | 201.9 | 208.9 | 235.6 | 307.1 | 357.5 |
| Surface roughness (Rt), microns (prototype) | 135 | 160 | 185 | 220 | 255 |
2.3. Cell Culture Processes and Imaging Techniques
3. Results and Discussion
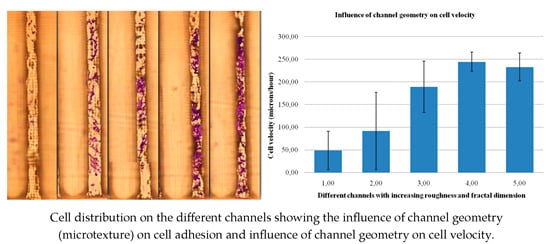
3.1. Impact of Surface Topography on Cell Adhesion


| Channel | Mean | Std. error | 95% Confidence interval | |
|---|---|---|---|---|
| 1 | 5,200 | 2,868 | 782 | 11,182 |
| 2 | 16,400 | 2,868 | 10,418 | 22,382 |
| 3 | 16,200 | 2,868 | 10,218 | 22,182 |
| 4 | 30,200 | 2,868 | 24,218 | 36,182 |
| 5 | 37,800 | 2,868 | 31,818 | 43,782 |
| Tukey HSD | Mean difference (I-J) | Std. Error | Significant | 95% Confidence interval | ||
|---|---|---|---|---|---|---|
| (I) Chanel | (J) Chanel | Lower bound | Upper bound | |||
| 1 | 2 | −11.20 | 4.056 | 0.079 | −23.34 | 0.94 |
| 3 | −11.00 | 4.056 | 0.087 | −23.14 | 1.14 | |
| 4 | −25.00 * | 4.056 | 0.000 | −37.14 | −12.86 | |
| 5 | −32.60 * | 4.056 | 0.000 | −44.74 | −20,46 | |
| 2 | 1 | 11.20 | 4.056 | 0.079 | −0.94 | 23.34 |
| 3 | 0.20 | 4.056 | 1,000 | −11.94 | 12.34 | |
| 4 | −13.80 * | 4.056 | 0.021 | −25.94 | −1.66 | |
| 5 | −21.40 * | 4.056 | 0.000 | −33.54 | −9.26 | |
| 3 | 1 | 11.00 | 4.056 | 0.087 | −1.14 | 23.14 |
| 2 | −0.20 | 4.056 | 1,000 | −12.34 | 11.94 | |
| 4 | −14.00 | 4.056 | 0.019 | −26.14 | −1.86 | |
| 5 | −21.60 | 4.056 | 0.000 | −33.74 | −9.46 | |
| 4 | 1 | 25.00 * | 4.056 | 0.000 | 12.86 | 37.14 |
| 2 | 13.80 * | 4.056 | 0.021 | 1.66 | 25.94 | |
| 3 | 14.00 * | 4.056 | 0.019 | 1.86 | 36.14 | |
| 5 | −7.60 | 4.056 | 0.362 | −19.74 | 4.54 | |
| 5 | 1 | 32.60 * | 4.056 | 0.000 | 20,46 | 44.74 |
| 2 | 21.40 * | 4.056 | 0.000 | 9.26 | 33.54 | |
| 3 | 21.60 * | 4.056 | 0.000 | 9.46 | 33.74 | |
| 4 | 7.60 | 4.056 | 0.362 | −4.54 | 19.74 | |
3.2. Impact of Surface Topography on Cell Motility and Behavior





| Channel | Mean | Std. Error | 95% Confidence Interval | |
|---|---|---|---|---|
| 1 | 19,600 | 6,610 | 6,286 | 32,914 |
| 2 | 36,667 | 6,610 | 23,353 | 49,981 |
| 3 | 75,733 | 6,610 | 62,419 | 89,047 |
| 4 | 97,800 | 6,610 | 84,486 | 111,114 |
| 5 | 93,200 | 6,610 | 79,886 | 106,514 |
| Tukey HSD | Mean Difference (I-J) | Std. Error | 95% Confidence Interval | Significant | ||
|---|---|---|---|---|---|---|
| (I) Chanel | (J) Chanel | Lower Bound | Upper Bound | |||
| 1 | 2 | −17.0667 | 9.34861 | 0.372 | −43.6303 | 9.4969 |
| 3 | −56.1333 * | 9.34861 | 0.000 | −82.6969 | −29.5697 | |
| 4 | −78.2000 * | 9.34861 | 0.000 | −104.7636 | −51.6364 | |
| 5 | −73.6000 * | 9.34861 | 0.000 | −100.1636 | −47.0364 | |
| 2 | 1 | 17.0667 | 9.34861 | 0.372 | −9.4969 | 43.6303 |
| 3 | −39.0667 * | 9.34861 | 0.001 | −65.6303 | −12.5031 | |
| 4 | −61.1333 * | 9.34861 | 0.000 | −87.6969 | −34.5697 | |
| 5 | −56.5333 * | 9.34861 | 0.000 | −83.0969 | −29.9697 | |
| 3 | 1 | 56.1333 * | 9.34861 | 0.000 | 29.5697 | 82.6969 |
| 2 | 39.0667 * | 9.34861 | 0.001 | 12.5031 | 65.6303 | |
| 4 | −22.0667 | 9.34861 | 0.145 | −48.6303 | 4.4969 | |
| 5 | −17.4667 | 9.34861 | 0.349 | −44.0303 | 9.0969 | |
| 4 | 1 | 78.2000 * | 9.34861 | 0.000 | 51.6364 | 104.7636 |
| 2 | 61.1333 * | 9.34861 | 0.000 | 34.5697 | 87.6969 | |
| 3 | 22.0667 | 9.34861 | 0.145 | −4.4969 | 48.6303 | |
| 5 | 4.6000 | 9.34861 | 0.988 | −21.9636 | 31.1636 | |
| 5 | 1 | 73.6000 * | 9.34861 | 0.000 | 47.0364 | 100.1636 |
| 2 | 56.5333 * | 9.34861 | 0.000 | 29.9697 | 83.0969 | |
| 3 | 17.4667 | 9.34861 | 0.349 | 9.0969 | 44.0303 | |
| 4 | −4.6000 | 9.34861 | 0.988 | −31.1636 | 21.9636 | |
4. Main Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Archard, J. Surface topography and tribology. Tribology 1974, 7, 213–220. [Google Scholar] [CrossRef]
- Bushan, B.; Israelachvili, J.; Landman, U. Nanotribology: Friction, wear and lubrication at the atomic scale. Nature 1995, 374, 607–616. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Buxboim, A.; Discher, D.E. Stem cells feel the difference. Nat. Methods 2010, 7, 695–697. [Google Scholar] [CrossRef] [PubMed]
- Berginski, M.; Hüpkes, J.; Schulte, M.; Schöpe, G.; Stiebig, H.; Rech, B. The effect of front ZnO:Al surface texture and optical transparency on efficient light trapping in silicon thin-film solar cells. J. Appl. Phys. 2007. [Google Scholar] [CrossRef]
- Briones, V.; Aguilera, J.M.; Brown, C. The effect of surface topography on color and gloss of chocolate samples. J. Food Eng. 2006, 77, 776–783. [Google Scholar] [CrossRef]
- Francesco, G.; Tirinato, L.; Battista, E.; Causa, F.; Liberale, C.; di Fabrizio, E.M.; Decuzzi, P. Cells preferentially grow on rough substrates. Biomaterials 2010, 31, 7205–7212. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Waters, M.S.; Farooque, T.M.; Young, M.F.; Simon, C.G., Jr. Freeform fabricated scaffolds with roughened struts that enhance both stem cell proliferation and differentiation by controlling cell shape. Biomaterials 2012, 33, 4022–4030. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.E.; Discher, D.E.; Shastri, V.P. Mechanical regulation of cells by materials and tissues. MRS Bull. 2010, 35, 578–583. [Google Scholar] [CrossRef]
- Chen, W.L.; Likhitpanichkul, M.; Ho, A.; Simmons, C.A. Integration of statistical modeling and high-content microscopy to systematically investigate cell-substrate interactions. Biomaterials 2010, 31, 2489–2497. [Google Scholar] [CrossRef] [PubMed]
- Buxboim, A.; Rajalgopal, K.; Brown, A.E.; Discher, D.E. How deeply cells feel: Methods for thin gels. J. Phys. Condens. Matter. 2010. [Google Scholar] [CrossRef] [PubMed]
- Madou, M.J. Fundamentals of Microfabrication: The Science of Miniaturization, 2nd ed.; CRC Press: New York, NY, USA, 2002. [Google Scholar]
- Chandra, P.; Lai, K.; Sunj, H.J.; Murthy, N.S.; Kohn, J. UV laser-ablated surface textures as potential regulator of cellular response. Biointerphases 2010, 5, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Aksay, I.A. Microchannel molding: A soft lithography-inspired approach to micrometer-scale patterning. J. Mater. Res. 2005, 20, 1995–2003. [Google Scholar] [CrossRef]
- Pulsifier, D.P.; Lakhtakia, A. Background and survey of bioreplication techniques. Bioinspir. Biomim. 2011. [Google Scholar] [CrossRef] [PubMed]
- Kwasny, W. Predicting properties of PVD and CVD coatings based on fractal quantities describing their surface. J. Achiev. Mater. Manuf. Eng. 2009, 37, 125–192. [Google Scholar]
- Jedlicka, S.S.; McKenzie, J.L.; Leavesley, S.L.; Little, K.M.; Webster, T.J.; Robinson, J.P.; Nivens, D.E.; Rickus, J.L. Sol–gel derived materials as substrates for neuronal differentiation: Effects of surface features and protein conformation. J. Mater. Chem. 2007, 16, 3221–3230. [Google Scholar] [CrossRef]
- Rahmawan, Y.; Xu, L.; Yang, S. Self-assembly of nanostructures towards transparent, superhydrophobic surfaces. J. Mater. Chem. A 2013, 1, 2955–2969. [Google Scholar] [CrossRef]
- Centola, M.; Rainer, A.; Spadaccio, C.; de Porcellinis, S.; Genovese, J.A.; Trombetta, M. Combining electrospinning and fused deposition modeling for the fabrication of a hybrid vascular graft. Biofabrication 2010. [Google Scholar] [CrossRef] [PubMed]
- Ballester-Beltran, J.; Lebourg, M.; Capella, H.; Diaz Lantada, A.; Salmerón-Sánchez, M. Robust fabrication of electrospun-like polymer mats to direct cell behaviour. Biofabrication 2014. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.M.; Morris, G.E.; Gould, T.W.A.; Ball, R.; Toumpaniari, S.; Harrington, H.; Dixon, J.E.; Shakesheff, K.M.; Segal, J.; Rose, F.R. A novel technique for the production of electrospun scaffolds with tailored 3D micro-patterns using additive manufacturing. Biofabrication 2014. [Google Scholar] [CrossRef] [PubMed]
- Gad-el-Hak, M. The MEMS Handbook; CRC Press: New York, NY, USA, 2003. [Google Scholar]
- Naik, V.M.; Mukherjee, R.; Majumder, A.; Sharma, A. Super functional materials: Creation and control of wetability, adhesion and optical effects by meso-texturing of surfaces. Curr. Trends Sci. Platin. Jubil. Spec. 2009, 129–148. [Google Scholar]
- Mandelbrot, B. The Fractal Geometry of Nature; W.H. Freeman & Co. Ltd.: San Francisco, CA, USA, 1982. [Google Scholar]
- Falconer, K. Fractal Geometry: Mathematical Foundations and Applications; John Wiley & Sons Ltd.: West Sussex, UK, 2003. [Google Scholar]
- Bückmann, T.; Stenger, N.; Kadic, M.; Kaschke, J.; Frölich, A.; Kennerknecht, T.; Eberl, C.; Thiel, M.; Wegener, M. Tailored 3D mechanical metamaterials made by dip-in direct-laser-writing optical lithography. Adv. Mater. 2012, 24, 2710–2714. [Google Scholar] [CrossRef] [PubMed]
- Röhrig, M.; Thiel, M.; Worgull, M.; Hölscher, H. Hierarchical structures: 3D direct laser writing of nano-microstructured hierarchical gecko-mimicking surface. Small 2012, 8, 3009–3015. [Google Scholar] [CrossRef] [PubMed]
- Clark, P.; Connolly, P.; Curtis, A.S.G.; Dow, J.A.T.; Wilkinson, C.D.W. Topographic control of cell behavior. Development 1987, 9, 439–448. [Google Scholar]
- Curtis, A.S.G.; Wilkinson, C.D.W. Topographical control of cells. Biomaterials 1997, 18, 1573–1583. [Google Scholar] [CrossRef]
- Flemming, R.G.; Murphy, C.J.; Abrams, G.A.; Goodman, S.L.; Nealey, P.F. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials 1999, 20, 573–588. [Google Scholar] [CrossRef]
- Crouch, A.S.; Miller, D.; Luebke, K.J.; Hu, W. Correlation of anisotropic cell behaviors with topographic aspect ratio. Biomaterials 2009, 30, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.; Medda, R.; Cheng, L.; Battista, E.; Scopelliti, P.E.; Milani, P.; Cavalcanti-Adam, E.A.; Decuzzi, P. Selective modulation of cell response on engineered fractal silicon substrates. Sci. Rep. 2013, 3, 1461. [Google Scholar] [CrossRef] [PubMed]
- Place, E.S.; Evans, N.; Stevens, M. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009, 8, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Díaz Lantada, A.; Pareja Sánchez, B.; Gómez Murillo, C.; Urbieta Sotillo, J. Fractals in tissue engineering: Toward biomimetic cell-culture matrices, microsystems and microstructured implants. Expert Rev. Med. Devices 2013, 10, 629–648. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.D.; Wang, F.W.; Matsumoto, K.; Yamada, K. One-dimensional topography underlies three-dimensional fibrillar cell migration. J. Cell Biol. 2009, 184, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Díaz Lantada, A.; Alarcón Iniesta, H.; Pareja Sánchez, B.; García-Ruíz, J.P. Free-form rapid-prototyped PDMS scaffolds incorporating growth factors promote chondrogenesis. Adv. Mater. Sci. Eng. 2014. [Google Scholar] [CrossRef]
- Richardson, T.P.; Peters, M.C.; Ennett, A.B.; Mooney, D.J. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001, 19, 1029. [Google Scholar] [CrossRef] [PubMed]
- Perets, A.; Baruch, Y.; Weisbuch, F.; Shoshany, G.; Neufeld, V.; Cohen, S. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J. Biomed. Mater. Res. A 2003, 65, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Rücker, M.; Jensen, G.; Carvalho, C.; Mülhaupt, R.; Gellrich, N.C.; Menger, M.D. Incorporation of growth factor containing Matrigel promotes vascularization of porous PLGA scaffolds. J. Biomed. Mater. Res. A 2008, 85, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Díaz Lantada, A. Handbook on Advanced Design and Manufacturing Technologies for Biomedical Devices; Springer: New York, NY, USA, 2013. [Google Scholar]
- Díaz Lantada, A.; Endrino, J.L.; Mosquera, A.A.; Lafont, P. Design and rapid prototyping of DLC coated fractal surfaces for tissue engineering applications. J. Phys. Conf. Ser. 2010. [Google Scholar] [CrossRef]
- Hengsbach, S.; Díaz Lantada, A. Rapid prototyping of multi-scale biomedical microdevices by combining additive manufacturing technologies. Biomed. Microdevices 2014, 16, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Díaz Lantada, A.; Piotter, V.; Plewa, K.; Barié, N.; Guttmann, M.; Wissmann, M. Towards mass production of microtextured microdevices: Linking rapid prototyping with micro injection molding. Int. J. Adv. Manuf. Technol. 2014. [Google Scholar] [CrossRef]
- Lennon, D.P.; Haynesworth, S.E.; Bruder, S.P.; Jaiswal, N.; Caplan, A.I. Human and animal mesenchymal progenitor cells from bone marrow: Identification of serum for optimal selection and proliferation. In vitro Cell. Dev. Biol. 2006, 32, 602–611. [Google Scholar] [CrossRef]
- Ogueta, S.; Muñoz, J.; Obregon, E.; Delgado-Baeza, E.; García-Ruiz, J.P. Prolactin is a component of the human sinovial liquid and modulates the growth and chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. Mol. Cell. Endocrinol. 2002, 190, 51–63. [Google Scholar] [CrossRef]
- Ynsa, M.D.; Dang, Z.Y.; Manso-Silvan, M.; Song, J.; Azimi, S.; Wu, J.F.; Liang, H.D.; Torres-Costa, V.; Punzon-Quijorna, E.; Breese, M.B.; et al. Reprogramming hMSCs morphology with silicon/porous silicon geometric micro-patterns. Biomed. Microdevices 2014, 16, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kuska, J.-P.; Zscharnack, M.; Loeffler, M.; Galle, J. Spatial organization of mesenchymal stem cells in vitro—Results from a new individual cell-based model with podia. PLoS ONE 2011, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jachetti, E.; di Renzo, C.; Meucci, S.; Nocchi, F.; Beltram, F.; Cecchini, M. Wharton’s jelly human mesenchymal stem cell contact guidance by noisy nanotopographies. Nat. Sci. Rep. 2014, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Peng, R.; Ding, J. Cell-material interactions revealed via material techniques of surface patterning. Adv. Mater. Mater. Views 2013, 25, 5257–5286. [Google Scholar] [CrossRef] [PubMed]
- Ostendorf, A.; Chichkov, B.N. Two-photon polymerization: A new approach to micromachining. Photon. Spectra 2006, 40, 72–80. [Google Scholar]
- Hermatsweiler, M. 3D-druck erobert die mikroskala. Laser Tech. J. 2013, 10, 55–57. [Google Scholar] [CrossRef]
- Longoni, S.; Sartori, M. Fractal geometry of nature (bone) may inspire medical devices shape. Nat. Prec. 2010, 1, 1–16. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz Lantada, A.; Alarcón Iniesta, H.; García-Ruíz, J.P. Multi-Channeled Polymeric Microsystem for Studying the Impact of Surface Topography on Cell Adhesion and Motility. Polymers 2015, 7, 2371-2388. https://doi.org/10.3390/polym7111519
Diaz Lantada A, Alarcón Iniesta H, García-Ruíz JP. Multi-Channeled Polymeric Microsystem for Studying the Impact of Surface Topography on Cell Adhesion and Motility. Polymers. 2015; 7(11):2371-2388. https://doi.org/10.3390/polym7111519
Chicago/Turabian StyleDiaz Lantada, Andres, Hernán Alarcón Iniesta, and Josefa Predestinación García-Ruíz. 2015. "Multi-Channeled Polymeric Microsystem for Studying the Impact of Surface Topography on Cell Adhesion and Motility" Polymers 7, no. 11: 2371-2388. https://doi.org/10.3390/polym7111519
APA StyleDiaz Lantada, A., Alarcón Iniesta, H., & García-Ruíz, J. P. (2015). Multi-Channeled Polymeric Microsystem for Studying the Impact of Surface Topography on Cell Adhesion and Motility. Polymers, 7(11), 2371-2388. https://doi.org/10.3390/polym7111519