Effect of Plasticizer Type and Concentration on Tensile, Thermal and Barrier Properties of Biodegradable Films Based on Sugar Palm (Arenga pinnata) Starch
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
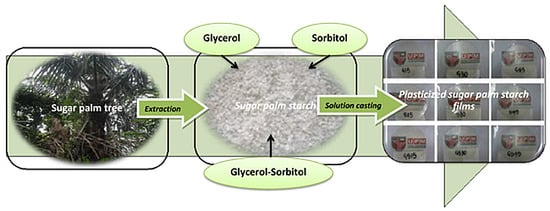
2.2. Film Preparation
2.3. Tensile Properties
2.4. Thermal-Gravimetric Analysis (TGA)
2.5. Differential Scanning Calorimetry (DSC)
2.6. Water Vapour Permeability (WVP)
3. Results and Discussion
3.1. Mechanical Properties
3.1.1. Tensile Strength of Sugar Palm Starch (SPS) Plasticized Films

3.1.2. Elongation at Break of SPS Plasticized Films


3.2. Thermal-Gravimetric Analysis (TGA)

3.3. Glass Transition Temperature (Tg)
| Samples | Type of Plasticizer | Plasticizer Content (%) | Tg (°C) | WVP × 10−10(g·s−1·m−1·Pa−1) |
|---|---|---|---|---|
| SPS | – | 0 | 145.19 | – |
| G15 | Glycerol | 15 | 139.77 | 5.820 ± 0.01 |
| G30 | 30 | 138.71 | 6.642 ± 0.07 | |
| G45 | 45 | 138.51 | 8.700 ± 0.01 | |
| S15 | Sorbitol | 15 | 141.65 | 4.855 ± 0.03 |
| S30 | 30 | 139.59 | 5.824 ± 0.01 | |
| S45 | 45 | 138.54 | 6.180 ± 0.02 | |
| GS15 | Glycerol-Sorbitol | 15 | 137.42 | 5.561 ± 0.04 |
| GS30 | 30 | 137 | 6.360 ± 0.01 | |
| GS45 | 45 | 123.46 | 8.514 ± 0.02 |
3.4. Water Vapor Permeability (WVP)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, M.; Li, D.; Wang, L.-J.; Adhikari, B. Creep behavior of starch-based nanocomposite films with cellulose nanofibrils. Carbohydr. Polym. 2015, 117, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Savadekar, N.R.; Mhaske, S.T. Synthesis of nano cellulose fibers and effect on thermoplastics starch based films. Carbohydr. Polym. 2012, 89, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Dang, K.M.; Yoksan, R. Development of thermoplastic starch blown film by incorporating plasticized chitosan. Carbohydr. Polym. 2015, 115, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Qiu, C.; Xiong, L.; Sun, Q. Characterisation of corn starch-based films reinforced with taro starch nanoparticles. Food Chem. 2015, 174, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Yakimets, I.; Paes, S.S.; Wellner, N.; Smith, A.C.; Wilson, R.H.; Mitchell, J.R. Effect of water content on the structural reorganization and elastic properties of biopolymer films: A comparative study. Biomacromolecules 2007, 8, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Muscat, D.; Adhikari, B.; Adhikari, R.; Chaudhary, D.S. Comparative study of film forming behaviour of low and high amylose starches using glycerol and xylitol as plasticizers. J. Food Eng. 2012, 109, 189–201. [Google Scholar] [CrossRef]
- López, O.V.; Lecot, C.J.; Zaritzky, N.E.; García, M.A. Biodegradable packages development from starch based heat sealable films. J. Food Eng. 2011, 105, 254–263. [Google Scholar] [CrossRef]
- Moreno, O.; Pastor, C.; Muller, J.; Atarés, L.; González, C.; Chiralt, A. Physical and bioactive properties of corn starch—Buttermilk edible films. J. Food Eng. 2014, 141, 27–36. [Google Scholar] [CrossRef]
- Da Rosa Zavareze, E.; Pinto, V.Z.; Klein, B.; El Halal, S.L.M.; Elias, M.C.; Prentice-Hernández, C.; Dias, A.R.G. Development of oxidised and heat–moisture treated potato starch film. Food Chem. 2012, 132, 344–350. [Google Scholar] [CrossRef]
- Talja, R.A.; Helén, H.; Roos, Y.H.; Jouppila, K. Effect of various polyols and polyol contents on physical and mechanical properties of potato starch-based films. Carbohydr. Polym. 2007, 67, 288–295. [Google Scholar] [CrossRef]
- Hu, G.; Chen, J.; Gao, J. Preparation and characteristics of oxidized potato starch films. Carbohydr. Polym. 2009, 76, 291–298. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Gonçalves, J.R.; El Halal, S.L.M.; Pinto, V.Z.; Dias, A.R.G.; Jacques, A.C.; Zavareze, E.D.R. Oxidation of potato starch with different sodium hypochlorite concentrations and its effect on biodegradable films. LWT Food Sci. Technol. 2015, 60, 714–720. [Google Scholar] [CrossRef]
- Cyras, V.P.; Zenklusen, M.C.T.; Vazquez, A. Relationship between structure and properties of modified potato starch biodegradable films. J. Appl. Polym. Sci. 2006, 101, 4313–4319. [Google Scholar] [CrossRef]
- Luk, E.; Sandoval, A.J.; Cova, A.; Müller, A.J. Anti-plasticization of cassava starch by complexing fatty acids. Carbohydr. Polym. 2013, 98, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.R.G.; da Rosa Zavareze, E.; Helbig, E.; de Moura, F.A.; Vargas, C.G.; Ciacco, C.F. Oxidation of fermented cassava starch using hydrogen peroxide. Carbohydr. Polym. 2011, 86, 185–191. [Google Scholar] [CrossRef]
- Klein, B.; Vanier, N.L.; Moomand, K.; Pinto, V.Z.; Colussi, R.; da Rosa Zavareze, E.; Dias, A.R.G. Ozone oxidation of cassava starch in aqueous solution at different pH. Food Chem. 2014, 155, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Espinel Villacrés, R.; Flores, S.K.; Gerschenson, L.N. Biopolymeric antimicrobial films: Study of the influence of hydroxypropyl methylcellulose, tapioca starch and glycerol contents on physical properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 36, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.; Benze, R.; Ferrão, E.S.; Ditch, C.; Coelho, A.C.V.; Tadini, C.C. Cassava starch biodegradable films: In fluence of glycerol and clay nanoparticles content on tensile and barrier properties and glass transition temperature. LWT Food Sci. Technol. 2012, 46, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Maran, J.P.; Sivakumar, V.; Sridhar, R.; Thirugnanasambandham, K. Development of model for barrier and optical properties of tapioca starch based edible films. Carbohydr. Polym. 2013, 92, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.M.O.; Yamashita, F.; Laurindo, J.B. Evaluation of the effects of glycerol and sorbitol concentration and water activity on the water barrier properties of cassava starch films through a solubility approach. Carbohydr. Polym. 2008, 72, 82–87. [Google Scholar] [CrossRef]
- Zamudio-Flores, P.B.; Vargas-Torres, A.; Pérez-González, J.; Bosquez-Molina, E.; Bello-Pérez, L.A. Films prepared with oxidized banana starch: Mechanical and barrier properties. Starch 2006, 58, 274–282. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Morales, N.J.; Pérez, E.; Tapia, M.S.; Famá, L. Physico-chemical properties of edible films derived from native and phosphated cush-cush yam and cassava starches. Food Packag. Shelf Life 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Mali, S.; Grossmann, M.V.E.; Garcı́a, M.; Martino, M.N.; Zaritzky, N.E. Barrier, mechanical and optical properties of plasticized yam starch films. Carbohydr. Polym. 2004, 56, 129–135. [Google Scholar] [CrossRef]
- Arockianathan, P.M.; Sekar, S.; Sankar, S.; Kumaran, B.; Sastry, T.P. Evaluation of biocomposite films containing alginate and sago starch impregnated with silver nano particles. Carbohydr. Polym. 2012, 90, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Nadiha, M.Z.N.; Fazilah, A.; Bhat, R.; Karim, A.A. Comparative susceptibilities of sago, potato and corn starches to alkali treatment. Food Chem. 2010, 121, 1053–1059. [Google Scholar] [CrossRef]
- Abdorreza, M.N.; Cheng, L.H.; Karim, A.A. Effects of plasticizers on thermal properties and heat sealability of sago starch films. Food Hydrocoll. 2011, 25, 56–60. [Google Scholar] [CrossRef]
- Laohakunjit, N.; Noomhorm, A. Effect of plasticizers on mechanical and barrier properties of rice starch film. Starch 2004, 56, 348–356. [Google Scholar] [CrossRef]
- Bourtoom, T.; Chinnan, M.S. Preparation and properties of rice starch–chitosan blend biodegradable film. LWT Food Sci. Technol. 2008, 41, 1633–1641. [Google Scholar] [CrossRef]
- Wittaya, T. Microcomposites of rice starch film reinforced with microcrystalline cellulose from palm pressed fiber. Int. Food Res. J. 2009, 16, 493–500. [Google Scholar]
- Dias, A.B.; Müller, C.M.O.; Larotonda, F.D.S.; Laurindo, J.B. Biodegradable films based on rice starch and rice flour. J. Cereal Sci. 2010, 51, 213–219. [Google Scholar] [CrossRef]
- Wu, M.; Wang, L.; Li, D.; Mao, Z.; Adhikari, B. Effect of flaxseed meal on the dynamic mechanical properties of starch-based films. J. Food Eng. 2013, 118, 365–370. [Google Scholar] [CrossRef]
- Krogars, K.; Heinämäki, J.; Karjalainen, M.; Niskanen, A.; Leskelä, M.; Yliruusi, J. Enhanced stability of rubbery amylose-rich maize starch films plasticized with a combination of sorbitol and glycerol. Int. J. Pharm. 2003, 251, 205–208. [Google Scholar] [CrossRef]
- Reis, K.C.; Pereira, J.; Smith, A.C.; Carvalho, C.W.P.; Wellner, N.; Yakimets, I. Characterization of polyhydroxybutyrate-hydroxyvalerate (PHB-HV)/maize starch blend films. J. Food Eng. 2008, 89, 361–369. [Google Scholar] [CrossRef]
- Xie, F.; Flanagan, B.M.; Li, M.; Sangwan, P.; Truss, R.W.; Halley, P.J.; Strounina, E.V.; Whittaker, A.K.; Gidley, M.J.; Dean, K.M.; et al. Characteristics of starch-based films plasticised by glycerol and by the ionic liquid 1-ethyl-3-methylimidazolium acetate: A comparative study. Carbohydr. Polym. 2014, 111, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Li, Y. Effects of glycerol and storage relative humidity on the properties of kudzu starch-based edible films. Starch 2014, 66, 524–532. [Google Scholar] [CrossRef]
- Wu, Y.; Geng, F.; Chang, P.R.; Yu, J.; Ma, X. Effect of agar on the microstructure and performance of potato starch film. Carbohydr. Polym. 2009, 76, 299–304. [Google Scholar] [CrossRef]
- The, D.P.; Debeaufort, F.; Voilley, A.; Luu, D. Biopolymer interactions affect the functional properties of edible films based on agar, cassava starch and arabinoxylan blends. J. Food Eng. 2009, 90, 548–558. [Google Scholar]
- Tian, H.; Xu, G.; Yang, B.; Guo, G. Microstructure and mechanical properties of soy protein/agar blend films: Effect of composition and processing methods. J. Food Eng. 2011, 107, 21–26. [Google Scholar] [CrossRef]
- Rhim, J.-W. Effect of clay contents on mechanical and water vapor barrier properties of agar-based nanocomposite films. Carbohydr. Polym. 2011, 86, 691–699. [Google Scholar] [CrossRef]
- Sahari, J.; Sapuan, S.M.; Ismarrubie, Z.N.; Rahman, M.Z.A. Physical and chemical properties of different morphological parts of sugar palm fibres. Fibres Text. East. Eur. 2012, 2, 21–24. [Google Scholar]
- Ishak, M.R.; Sapuan, S.M.; Leman, Z.; Rahman, M.Z.A.; Anwar, U.M.K.; Siregar, J.P. Sugar palm (Arenga pinnata): Its fibres, polymers and composites. Carbohydr. Polym. 2013, 91, 699–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahari, J.; Sapuan, S.M.; Zainudin, E.S.; Maleque, M.A. A new approach to use Arenga pinnata as sustainable biopolymer: Effects of plasticizers on physical properties. Procedia Chem. 2012, 254–259. [Google Scholar] [CrossRef]
- Sahari, J.; Sapuan, S.M.; Zainudin, E.S.; Maleque, M.A. Thermo-mechanical behaviors of thermoplastic starch derived from sugar palm tree (Arenga pinnata). Carbohydr. Polym. 2013, 92, 1711–1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wina, E.; Evans, A.J.; Lowry, J.B. The composition of pith from the sago palms Metroxylon sagu and Arenga pinnata. J. Sci. Food Agric. 1986, 37, 352–358. [Google Scholar] [CrossRef]
- Adawiyah, D.R.; Sasaki, T.; Kohyama, K. Characterization of arenga starch in comparison with sago starch. Carbohydr. Polym. 2013, 92, 2306–2313. [Google Scholar] [CrossRef] [PubMed]
- Sahari, J.; Sapuan, S.M.; Zainudin, E.S.; Maleque, M.A. Physico-chemical and thermal properties of starch derived from sugar palm tree (Arenga pinnata). Asian J. Chem. 2014, 26, 955–959. [Google Scholar]
- Yu, F.; Prashantha, K.; Soulestin, J.; Lacrampe, M.-F.; Krawczak, P. Plasticized-starch/poly (ethylene oxide) blends prepared by extrusion. Carbohydr. Polym. 2013, 91, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Blácido, D.R.; do Amaral Sobral, P.J.; Menegalli, F.C. Optimization of amaranth flour films plasticized with glycerol and sorbitol by multi-response analysis. LWT Food Sci. Technol. 2011, 44, 1731–1738. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Aguirre, A.; Borneo, R.; León, A.E. Properties of triticale protein films and their relation to plasticizing–antiplasticizing effects of glycerol and sorbitol. Ind. Crops Prod. 2013, 50, 297–303. [Google Scholar] [CrossRef]
- Razavi, S.M.A.; Amini, A.M.; Zahedi, Y. Characterisation of a new biodegradable edible film based on sage seed gum: Influence of plasticiser type and concentration. Food Hydrocoll. 2015, 43, 290–298. [Google Scholar] [CrossRef]
- Smits, A.L.M.; Kruiskamp, P.H.; van Soest, J.J.G.; Vliegenthart, J.F.G. Interaction between dry starch and plasticisers glycerol or ethylene glycol, measured by differential scanning calorimetry and solid state NMR spectroscopy. Carbohydr. Polym. 2003, 53, 409–416. [Google Scholar] [CrossRef]
- Rodríguez, M.; Oses, J.; Ziani, K.; Mate, J.I. Combined effect of plasticizers and surfactants on the physical properties of starch based edible films. Food Res. Int. 2006, 39, 840–846. [Google Scholar] [CrossRef]
- Garcia, M.A.; Martino, M.N.; Zaritzky, N.E. Lipid addition to improve barrier properties of edible starch-based films and coatings. J. Food Sci. 2000, 65, 941–944. [Google Scholar] [CrossRef]
- Fishman, M.L.; Coffin, D.R.; Konstance, R.P.; Onwulata, C.I. Extrusion of pectin/starch blends plasticized with glycerol. Carbohydr. Polym. 2000, 41, 317–325. [Google Scholar] [CrossRef]
- Bergo, P.V.A.; Carvalho, R.A.; Sobral, P.J.A.; dos Santos, R.M.C.; da Silva, F.B.R.; Prison, J.M.; Solorza-Feria, J.; Habitante, A. Physical properties of edible films based on cassava starch as affected by the plasticizer concentration. Packag. Technol. Sci. 2008, 21, 85–89. [Google Scholar] [CrossRef]
- Mali, S.; Sakanaka, L.S.; Yamashita, F.; Grossmann, M.V.E. Water sorption and mechanical properties of cassava starch films and their relation to plasticizing effect. Carbohydr. Polym. 2005, 60, 283–289. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Heikkinen, S.; Soovre, A.; Peura, M.; Serimaa, R.; Talja, R.A.; Helén, H.; Hyvönen, L.; Tenkanen, M. Films from oat spelt arabinoxylan plasticized with glycerol and sorbitol. J. Appl. Polym. Sci. 2009, 14, 457–466. [Google Scholar] [CrossRef]
- Kuutti, P.; Peltonen, L.; Myllärinen, J.; Teleman, P.; Forssell, O. AFM in studies of thermoplastic starches during ageing. Carbohydr. Polym. 1998, 37, 7–12. [Google Scholar] [CrossRef]
- Suyatma, V.; Tighzert, N.E.; Copinet, L.; Coma, A. Effects of hydrophilic plasticizers on mechanical, thermal, and surface properties of chitosan films. J. Agric. Food Chem. 2005, 50, 3950–3957. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, E.; Chaudhary, B.; Clerfeuille, D.S. Effect of plasticizers on the moisture migration behavior of low-amylose starch films during drying. Dry. Technol. 2010, 28, 468–480. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Wang, L.J.; Li, D.; Wei, Q.; Adhikari, B. Effects of high-pressure homogenization on the properties of starch-plasticizer dispersions and their films. Carbohydr. Polym. 2011, 86, 202–207. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A. Physical, mechanical, barrier, and thermal properties of polyol-plasticized biodegradable edible film made from kefiran. Carbohydr. Polym. 2011, 84, 477–483. [Google Scholar] [CrossRef]
- Imran, M.; El-Fahmy, S.; Revol-Junelles, S.; Desobry, A.M. Cellulose derivative based active coatings: Effects of nisin and plasticizer on physico-chemical and antimicrobial properties of hydroxypropyl methylcellulose films. Carbohydr. Polym. 2010, 81, 219–225. [Google Scholar] [CrossRef]
- Jouki, M.; Khazaei, N.; Ghasemlou, M.; HadiNezhad, M. Effect of glycerol concentration on edible film production from cress seed carbohydrate gum. Carbohydr. Polym. 2013, 96, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Thakhiew, W.; Devahastin, S.; Soponronnarit, S. Effects of drying methods and plasticizer concentration on some physical and mechanical properties of edible chitosan films. J. Food Eng. 2010, 99, 216–224. [Google Scholar] [CrossRef]
- Turhan, K.N.; Şahbaz, F. Water vapor permeability, tensile properties and solubility of methylcellulose-based edible films. J. Food Eng. 2004, 61, 459–466. [Google Scholar] [CrossRef]
- Tapia-Blácido, D.R.; Sobral, P.J.D.A.; Menegalli, F.C. Effect of drying conditions and plasticizer type on some physical and mechanical properties of amaranth flour films. LWT Food Sci. Technol. 2013, 50, 392–400. [Google Scholar] [CrossRef]
- Pushpadass, H.A.; Bhandari, P.; Hanna, M.A. Effects of LDPE and glycerol contents and compounding on the microstructure and properties of starch composite films. Carbohydr. Polym. 2010, 82, 1082–1089. [Google Scholar] [CrossRef]
- Dai, H.; Chang, P.R.; Yu, J.; Ma, X. N,N-Bis(2-hydroxyethyl) formamide as a new plasticizer for thermoplastic starch. Starch 2008, 60, 676–684. [Google Scholar] [CrossRef]
- Mehyar, G.F.; Han, J.H. Physical and mechanical properties of high-amylose rice and pea starch films as affected by relative humidity and plasticizer. J. Food Sci. 2004, 69, E449–E454. [Google Scholar] [CrossRef]
- Müller, C.M.O.; Laurindo, J.B.; Yamashita, F. Effect of cellulose fibers addition on the mechanical properties and water vapor barrier of starch-based films. Food Hydrocoll. 2009, 23, 1328–1333. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Tapia, M.S.; Pérez, E.; Famá, L. Structural and mechanical properties of edible films made from native and modified cush–cush yam and cassava starch. Food Hydrocoll. 2015, 45, 211–217. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, J.H. Plasticization of pea starch films with monosaccharides and polyols. J. Food Sci. 2006, 71, E253–E261. [Google Scholar] [CrossRef]
- De Moraes, J.O. Propriedades de filmes de amido incorporados de nanoargilas e fibras de celulose. Ph.D., Universidade Federal de Santa Catarina, 2009. [Google Scholar]
- Kurt, A.; Kahyaoglu, T. Characterization of a new biodegradable edible film made from salep glucomannan. Carbohydr. Polym. 2014, 104, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Suppakul, P.; Chalernsook, B.; Ratisuthawat, B.; Prapasitthi, S.; Munchukangwan, N. Empirical modeling of moisture sorption characteristics and mechanical andbarrier properties of cassava flour film and their relation to plasticizing–antiplasticizing effects. LWT Food Sci. Technol. 2013, 50, 290–297. [Google Scholar] [CrossRef]
- Mali, S.; Grossmann, M.V.E.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Effects of controlled storage on thermal, mechanical and barrier properties of plasticized films from different starch sources. J. Food Eng. 2006, 75, 453–460. [Google Scholar] [CrossRef]
- Zhang, Y.; Rempel, C. Retrogradation and antiplasticization of thermoplastic starch. In Thermoplastic Elastomers; El-Sonbati, A., Ed.; InTech Open Access Publisher: Rijeka, Croatia, 2012; pp. 118–119. [Google Scholar]
- Gaudin, S.; Lourdin, D.; Forssell, P.M.; Colonna, P. Antiplasticisation and oxygen permeability of starch–sorbitol films. Carbohydr. Polym. 2000, 43, 33–37. [Google Scholar] [CrossRef]
- Chang, Y.P.; Karim, A.A.; Seow, C.C. Interactive plasticizing–antiplasticizing effects of water and glycerol on the tensile properties of tapioca starch films. Food Hydrocoll. 2006, 20, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Rempel, C.; Liu, Q. Thermoplastic starch processing and characteristics—A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1353–1370. [Google Scholar] [CrossRef] [PubMed]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of plasticizer type and concentration on physical properties of sugar palm starch (Arenga pinnata) films. Ind. Crops Prod. 2015. under review. [Google Scholar]
- Wang, J.; Jiang, N.; Jiang, H. The high-temperatures bonding of graphite/ceramics by organ resin matrix adhesive. Int. J. Adhes. Adhes. 2006, 26, 532–536. [Google Scholar] [CrossRef]
- Rajan, A.; Prasad, V.S.; Abraham, T.E. Enzymatic esterification of starch using recovered coconut oil. Int. J. Biol. Macromol. 2006, 39, 265–272. [Google Scholar] [CrossRef] [PubMed]
- García, N.L.; Famá, L.; Dufresne, A.; Aranguren, M.; Goyanes, S. A comparison between the physico-chemical properties of tuber and cereal starches. Food Res. Int. 2009, 42, 976–982. [Google Scholar] [CrossRef]
- Nascimento, T.A.; Calado, V.; Carvalho, C.W.P. Development and characterization of flexible film based on starch and passion fruit mesocarp flour with nanoparticles. Food Res. Int. 2012, 49, 588–595. [Google Scholar] [CrossRef]
- Chiumarelli, M.; Hubinger, M.D. Evaluation of edible films and coatings formulated with cassava starch, glycerol, carnauba wax and stearic acid. Food Hydrocoll. 2014, 38, 20–27. [Google Scholar] [CrossRef]
- Cuq, B.; Gontard, N.; Aymard, C.; Guilbert, S. Relative humidity and temperature effects on mechanical and water vapor barrier properties of myofibrillar protein-based films. Polym. Gels Netw. 1997, 5, 1–15. [Google Scholar] [CrossRef]
- López, O.V.; Castillo, L.A.; García, M.A.; Villar, M.A.; Barbosa, S.E. Food packaging bags based on thermoplastic corn starch reinforced with talc nanoparticles. Food Hydrocoll. 2015, 43, 18–24. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of Plasticizer Type and Concentration on Tensile, Thermal and Barrier Properties of Biodegradable Films Based on Sugar Palm (Arenga pinnata) Starch. Polymers 2015, 7, 1106-1124. https://doi.org/10.3390/polym7061106
Sanyang ML, Sapuan SM, Jawaid M, Ishak MR, Sahari J. Effect of Plasticizer Type and Concentration on Tensile, Thermal and Barrier Properties of Biodegradable Films Based on Sugar Palm (Arenga pinnata) Starch. Polymers. 2015; 7(6):1106-1124. https://doi.org/10.3390/polym7061106
Chicago/Turabian StyleSanyang, Muhammed L., Salit M. Sapuan, Mohammad Jawaid, Mohamad R. Ishak, and Japar Sahari. 2015. "Effect of Plasticizer Type and Concentration on Tensile, Thermal and Barrier Properties of Biodegradable Films Based on Sugar Palm (Arenga pinnata) Starch" Polymers 7, no. 6: 1106-1124. https://doi.org/10.3390/polym7061106
APA StyleSanyang, M. L., Sapuan, S. M., Jawaid, M., Ishak, M. R., & Sahari, J. (2015). Effect of Plasticizer Type and Concentration on Tensile, Thermal and Barrier Properties of Biodegradable Films Based on Sugar Palm (Arenga pinnata) Starch. Polymers, 7(6), 1106-1124. https://doi.org/10.3390/polym7061106