Influences of Alkyl and Aryl Substituents on Iminopyridine Fe(II)- and Co(II)-Catalyzed Isoprene Polymerization
Abstract
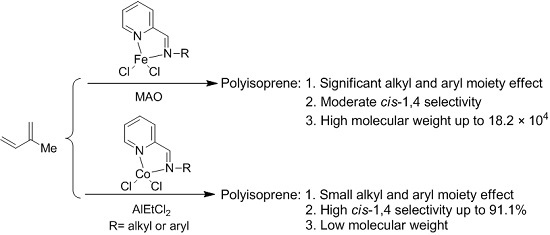
:1. Introduction
2. Experimental Section
2.1. General Information
2.2. General Procedure for the Synthesis of Ligands L1, L3, and L4
2.3. General Procedure for the Synthesis of Iron Complexes
2.4. General Procedure for the Synthesis of Cobalt Complexes
2.5. General Procedure for Isoprene Polymerization
2.6. Calculation of Microstructure Contents of Polyisoprenes
3. Results and Discussion
3.1. Synthesis and Characterization of the Iron and Cobalt Complexes
3.2. Isoprene Polymerization Studies
3.2.1. Polymerization of Isoprene with Iron Catalysts
3.2.2. Polymerization of Isoprene with Co(II) Catalysts
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Horne, S.E., Jr.; Kiehl, J.P.; Shipman, J.J.; Folt, V.L.; Gibbs, C.F. Ameripol SN-A cis-1,4-polyisoprene. Ind. Eng. Chem. 1956, 48, 784–791. [Google Scholar] [CrossRef]
- Natta, G. Progress in five years of research in stereospecific polymerization. SPE J. 1959, 53, 373–382. [Google Scholar]
- Proto, A.; Capacchione, C. Stereoselective Polymerization with Single-Site Catalysts; Baugh, L.S., Canich, J.A.M., Eds.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Ricci, G.; Sommazzi, A.; Masi, F.; Ricci, M.; Boglia, A.; Leone, G. Well-defined transition metal complexes with phosphorus and nitrogen ligands for 1,3-dienes polymerization. Coord. Chem. Rev. 2010, 254, 661–676. [Google Scholar] [CrossRef]
- Osakada, K.; Takeuchi, D. Coordination polymerization of dienes, allenes, and methylenecycloalkanes. Adv. Polym. Sci. 2004, 171, 137–194. [Google Scholar]
- Zhang, L.X.; Suzuki, T.; Luo, Y.; Nishiura, M.; Hou, Z.M. Cationic alkyl rare-earth metal complexes bearing an ancillary Bis(phosphinophenyl)amido ligand: A catalytic system for living cis-1,4-polymerization and copolymerization of isoprene and butadiene. Angew. Chem. Int. Ed. 2007, 46, 1909–1913. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Cui, D.M. Highly cis-1,4 selective polymerization of dienes with homogeneous Ziegler-Natta catalysts based on NCN-pincer rare earth metal dichloride precursors. J. Am. Chem. Soc. 2008, 130, 4984–4991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Nishiura, M.; Yuki, M.; Luo, Y.; Hou, Z.M. Isoprene polymerization with yttrium amidinate catalysts: Switchingthe regio-and stereoselectivity by addition of AlMe3. Angew. Chem. Int. Ed. 2008, 47, 2642–2645. [Google Scholar] [CrossRef] [PubMed]
- Li, D.F.; Li, S.H.; Cui, D.M.; Zhang, X.Q. β-diketiminato rare-earth metal complexes. Structures, catalysis, and active species for highly cis-1,4-selective polymerization of isoprene. Organometallics 2010, 29, 2186–2193. [Google Scholar] [CrossRef]
- Lv, K.; Cui, D.M. CCC-pincer bis(carbene) lanthanide dibromides. Catalysis on highlycis-1,4-selective polymerization of isoprene and active species. Organometallics 2010, 29, 2987–2993. [Google Scholar] [CrossRef]
- Nishiura, M.; Hou, Z.M. Novel polymerization catalysts and hydride clusters from rare-earth metal dialkyls. Nat. Chem. 2010, 2, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Milione, S.; Cuomo, C.; Capacchione, C.; Zannoni, C.; Grassi, A.; Proto, A. Stereoselective polymerization of conjugated dienes and styrene-butadiene copolymerization promoted by octahedral titanium catalyst. Macromolecules 2007, 40, 5638–5643. [Google Scholar] [CrossRef]
- Buonerba, A.; Fienga, M.; Milione, S.; Cuomo, C.; Grassi, A.; Proto, A.; Capacchione, C. Binary copolymerization of p-methylstyrene with butadiene and isoprene catalyzed by titanium compounds showing different stereoselectivity. Macromolecules 2013, 46, 8449–8457. [Google Scholar] [CrossRef]
- Proto, A.; Avagliano, A.; Saviello, D.; Ricciardi, R.; Capacchione, C. Living, isoselective polymerization of styrene and formation of stereoregular block copolymers via sequential monomer addition. Macromolecules 2010, 43, 5919–5921. [Google Scholar] [CrossRef]
- Capacchione, C.; Saviello, D.; Ricciardi, R.; Proto, A. Living, isoselective polymerization of 4-methyl-1,3-pentadiene and styrenic monomers and synthesis of highly stereoregular block copolymers via sequential monomer addition. Macromolecules 2011, 44, 7940–7947. [Google Scholar] [CrossRef]
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New Pd(II)- and Ni(II)-based catalysts for polymerization of ethylene and α-olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Nakamura, A.; Ito, S.; Nozaki, K. Coordination-insertion copolymerization of fundamental polar monomers. Chem. Rev. 2009, 109, 5215–5244. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.X. Coordination polymerization of polar vinyl monomers by single-site metal catalysts. Chem. Rev. 2009, 109, 5157–5214. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Cotts, P.M.; McCord, E.F.; McLain, S.J. Chain Walking: A new strategy to control polymer topology. Science 1999, 283, 2059–2061. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, T.; Klimovica, K.; LaPointe, A.M.; Keresztes, I.; Lobkovsky, E.B.; Daugulis, O.; Coates, G.W. Secondary alkene insertion and precision chain-walking: A new route to semicrystalline “polyethylene” from α‑olefins by combining two rare catalytic events. J. Am. Chem. Soc. 2014, 136, 7213–7216. [Google Scholar] [CrossRef] [PubMed]
- Takano, S.; Takeuchi, D.; Osakada, K.; Akamatsu, N.; Shishido, A. Dipalladium catalyst for olefin polymerization: Introduction of acrylate units into the main chain of branched polyethylene. Angew. Chem. Int. Ed. 2014, 53, 9246–9250. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.H.; Chen, C.L. (α-Diimine) palladium catalyzed ethylene polymerization and copolymerization with polar comonomers. Sci. China Chem. 2015, 58, 1663–1673. [Google Scholar] [CrossRef]
- Guo, L.H.; Dai, S.Y.; Sui, X.L.; Chen, C.L. Palladium and nickel catalyzed chain walking olefin polymerization and copolymerization. ACS Catal. 2016, 6, 428–441. [Google Scholar] [CrossRef]
- Guo, L.; Gao, H.; Guan, Q.; Hu, H.; Deng, J.; Liu, J.; Liu, F.; Wu, Q. Substituent effects of the backbone in α-diimine palladium catalysts on homo- and copolymerization of ethylene with methyl acrylate. Organometallics 2012, 31, 6054–6062. [Google Scholar] [CrossRef]
- Guo, L.H.; Sui, X.L.; Dai, S.Y.; Chen, C.L. Ligand electronic effects on α-diimine nickel(II) catalyzed ethylene polymerization. Polymers 2016, 8, 37. [Google Scholar] [CrossRef]
- Dai, S.Y.; Chen, C.L. Direct synthesis of functionalized high-molecular-weight polyethylene by copolymerization of ethylene with polar monomers. Angew. Chem. Int. Ed. 2016, 55, 13281–13285. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.Y.; Sui, X.L.; Chen, C.L. Synthesis of high molecular weight polyethylene using iminopyridyl nickel catalysts. Chem. Commun. 2016, 52, 9113–9116. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.K.; Zhao, M.H.; Chen, C.L. Influence of ligand second coordination sphere effects on the olefin (co)polymerization properties of α-diimine Pd(II) catalysts. Polym. Chem. 2016, 7, 3933–3938. [Google Scholar] [CrossRef]
- Hu, X.H.; Dai, S.Y.; Chen, C.L. Ethylene polymerization by salicylaldimine nickel(II) complexes containing dibenzhydryl moiety. Dalton Trans. 2016, 45, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.K.; Sui, X.L.; Pang, W.M.; Chen, C.L. Ethylene polymerization by xanthene bridged dinuclear α-diimine Ni(II) complexes. ChemCatChem 2016, 8, 434–440. [Google Scholar] [CrossRef]
- Chen, M.; Yang, B.P.; Chen, C.L. Redox-controlled olefin (co)polymerization catalyzed by ferrocene bridged phosphine-sulfonate palladium complexes. Angew. Chem. Int. Ed. 2015, 54, 15520–15524. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.Y.; Sui, X.L.; Chen, C.L. Highly robust Pd(II) α–diimine catalysts for slow-chain-walking polymerization of ethylene and copolymerization with methyl acrylate. Angew. Chem. Int. Ed. 2015, 54, 9948–9953. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.L.; Dai, S.Y.; Chen, C.L. Ethylene polymerization and copolymerization with polar monomers by cationic phosphine phosphonic amide palladium complexes. ACS Catal. 2015, 5, 5932–5937. [Google Scholar] [CrossRef]
- Chen, M.; Zou, W.P.; Cai, Z.G.; Chen, C.L. Norbornene homopolymerization and copolymerization with ethylene by phosphine-sulfonate nickel catalysts. Polym. Chem. 2015, 6, 2669–2676. [Google Scholar] [CrossRef]
- Ricci, G.; Battistella, M.; Porri, L. Chemoselectivity and stereospecificity of chromium(II) catalysts for 1,3-diene polymerization. Macromolecules 2001, 34, 5766–5769. [Google Scholar] [CrossRef]
- Bazzini, C.; Giarrusso, A.; Porri, L. Diethylbis(2,2′-bipyridine)iron/MAO. A very active and stereospecific catalyst for 1,3-diene polymerization. Macromol. Rapid Commun. 2002, 23, 922–927. [Google Scholar] [CrossRef]
- Ricci, G.; Morganti, D.; Sommazzi, A.; Santi, R.; Masi, F. Polymerization of 1,3-dienes with iron complexes based catalysts influence of the ligand on catalyst activity and stereospecificity. J. Mol. Catal. A Chem. 2003, 204–205, 287–293. [Google Scholar] [CrossRef]
- Bazzini, C.; Giarrusso, A.; Porri, L.; Pirozzi, B.; Napolitano, R. Synthesis and characterization of syndiotactic 3,4-polyisoprene prepared with diethylbis(2,2′-bipyridine)iron-MAO. Polymer 2004, 45, 2871–2875. [Google Scholar] [CrossRef]
- Ricci, G.; Forni, A.; Boglia, A.; Sonzogni, M. New chromium(II) bidentate phosphine complexes: Synthesis, characterization, and behavior in the polymerization of 1,3-butadiene. Organometallics 2004, 23, 3727–3732. [Google Scholar] [CrossRef]
- Ricci, G.; Forni, A.; Boglia, A.; Motta, T. Synthesis, structure, and butadiene polymerization behavior of alkylphosphine cobalt(II) complexes. J. Mol. Catal. A Chem. 2005, 226, 235–241. [Google Scholar] [CrossRef]
- Dai, Q.Q.; Jia, X.Y.; Yang, F.; Bai, C.X.; Hu, Y.M.; Zhang, X.Q. Iminopyridine-based cobalt(II) and nikel(II) complexes: Synthesis, characterization, and their catalytic behaviors for 1,3-butadiene polymerization. Polymers 2016, 8, 12. [Google Scholar] [CrossRef]
- Gong, D.R.; Dong, W.M.; Hu, Y.M.; Bi, J.F.; Zhang, X.Q.; Jiang, L.S. Syndiotactically enriched 1,2-selective polymerization of 1,3-butadiene initiated by iron catalysts based on a new class of donors. Polymer 2009, 50, 5980–5986. [Google Scholar] [CrossRef]
- Cámpora, J.; Tabla, L.O.; Palma, P.; Álvarez, E.; Lahoz, F.; Mereiter, K. Synthesis and catalytic activity of cationic allyl complexes of nickel stabilized by a single n-heterocyclic carbene ligand. Organometallcs 2006, 25, 3314–3316. [Google Scholar] [CrossRef]
- Ricci, G.; Forni, A.; Boglia, A.; Motta, T.; Zannoni, G.; Canetti, M.; Bertini, F. Synthesis and X-ray structure of CoCl2 (PiPrPh2)2. A new highly active and stereospecific catalyst for 1,2 polymerization of conjugated dienes when used in association with MAO. Macromolecules 2005, 38, 1064–1070. [Google Scholar] [CrossRef]
- Ricci, G.; Boglia, A.; Motta, T. Synthesis of new Cr(II) complexes with bidentate phosphine ligands and their behavior in the polymerization of butadiene influence of the phosphine bite angle on catalyst activity and stereoselectivity. J. Mol. Catal. A Chem. 2007, 267, 102–107. [Google Scholar] [CrossRef]
- Ricci, G.; Leone, G.; Boglia, A.; Boccia, A.C.; Zetta, L. cis-1,4-alt-3,4 polyisoprene: Synthesis and characterization. Macromolecules 2009, 42, 9263–9267. [Google Scholar] [CrossRef]
- Ricci, G.; Leone, G.; Boglia, A.; Bertini, F.; Boccia, A.C.; Zetta, L. Synthesis and characterization of isotactic 1,2-poly(E-3-methyl-1,3-pentadiene). Some remarks about the influence of monomer structure on polymerization stereoselectivity. Macromolecules 2009, 42, 3048–3056. [Google Scholar] [CrossRef]
- Ricci, G.; Motta, T.; Boglia, A.; Alberti, E.; Zetta, L.; Bertini, F.; Arosio, P.; Famulari, A.; Meille, S.V. Synthesis, characterization, and crystalline structure of syndiotactic 1,2-polypentadiene: The trans polymer. Macromolecules 2005, 38, 8345–8352. [Google Scholar] [CrossRef]
- Ricci, G.; Boglia, A.; Motta, T.; Bertini, F.; Boccia, A.C.; Zetta, L.; Alberti, E.; Famulari, A.; Arosio, P.; Meille, S.V. Synthesis and structural characterization of syndiotactic trans-1,2 and cis-1,2 polyhexadienes. J. Polym. Sci. A Polym. Chem. 2007, 45, 5339–5353. [Google Scholar] [CrossRef]
- He, A.H.; Wang, G.; Zhao, W.Z.; Jiang, X.B.; Yao, W.; Sun, W.H. High cis-1,4 polyisoprene or cis-1,4/3,4 binary polyisoprene synthesized using 2-(benzimidazolyl)-6-(1-(arylimino)ethyl)pyridine cobalt(II) dichlorides. Polym. Int. 2013, 62, 1758–1766. [Google Scholar] [CrossRef]
- Raynaud, J.; Wu, J.Y.; Ritter, T. Iron-catalyzed polymerization of isoprene and other 1,3-dienes. Angew. Chem. Int. Ed. 2012, 51, 11805–11808. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Stanzl, B.N.; Ritter, T. A Strategy for the synthesis of well-defined iron catalysts and application to regioselective diene hydrosilylation. J. Am. Chem. Soc. 2010, 132, 13214–13216. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.H.; Song, S.J.; Li, B.X.; Redshaw, C.; Hao, X.; Li, Y.S.; Wang, F.S. Ethylene polymerization by 2-iminopyridylnickel halide complexes: Synthesis, characterization and catalytic influence of the benzhydryl group. Dalton Trans. 2012, 41, 11999–12010. [Google Scholar] [CrossRef] [PubMed]
- Gibson, V.C.; O′Reilly, R.K.; Wass, D.F.; White, A.J.P.; Williams, D.J. Iron complexes bearing iminopyridine and aminopyridine ligands as catalysts for atom transfer radical polymerization. Dalton Trans. 2003. [Google Scholar] [CrossRef]
- Wang, B.L.; Cui, D.M.; Lv, K. Highly 3,4-selective living polymerization of isoprene with rare earth metal fluorenyl N-heterocyclic carbene precursors. Macromolecules 2008, 41, 1983–1988. [Google Scholar] [CrossRef]






| Entry | Complex | T (°C) | Yield (%) | Activity c | Mn d (×10−4) | PDI d | Microstructure c (%) e | |||
|---|---|---|---|---|---|---|---|---|---|---|
| cis-1,4 | trans-1,4 | cis/trans | 3,4 | |||||||
| 1 b | 2a | 25 | 83.4 | 7.1 | 0.18 | 4.70 | - | - | - | |
| 2 | 2a | 25 | 83.1 | 7.1 | 6.1 | 1.57 | 77.5 | 8.1 | 91:9 | 14.4 |
| 3 | 2a | −25 | 66.3 | 5.6 | 7.9 | 2.45 | 77.0 | 8.7 | 90:10 | 14.3 |
| 4 | 1a | 25 | 64.1 | 5.4 | 6.0 | 2.11 | 77.1 | 8.9 | 90:10 | 14.0 |
| 5 | 3a | 25 | 58.2 | 4.9 | 7.0 | 1.82 | 76.8 | 8.2 | 90:10 | 15.0 |
| 6 | 4a | 25 | 61.3 | 5.2 | 6.1 | 2.08 | 78.2 | 7.6 | 91:9 | 14.2 |
| 7 | 5a | −25 | 81.0 | 6.9 | 15.4 | 2.13 | 63.9 | 3.0 | 96:4 | 33.1 |
| 8 | 5a | 25 | 98.1 | 8.3 | 10.3 | 2.05 | 62.7 | 2.8 | 96:4 | 34.5 |
| 9 | 6a | 25 | 83.2 | 7.1 | 18.0 | 1.75 | 69.9 | 4.5 | 94:6 | 25.6 |
| 10 | 7a | 25 | 85.7 | 7.3 | 18.2 | 1.61 | 71.4 | 4.8 | 94:6 | 23.8 |
| Entry | Complex | Yield (%) | Activity b | Mn c (×10−3) | PDI c | Microstructure d (%) | |
|---|---|---|---|---|---|---|---|
| cis-1,4 | 3,4 | ||||||
| 1 | 2b | 78.2 | 6.6 | 1.4 | 7.97 | 91.1 | 8.9 |
| 2 | 3b | 76.9 | 6.5 | 1.5 | 4.76 | 90.8 | 9.2 |
| 3 | 5b | 97.3 | 8.3 | 1.7 | 8.05 | 88.1 | 11.9 |
| 4 | 6b | 94.9 | 8.1 | 1.8 | 9.38 | 89.7 | 10.3 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Jing, X.; Xiong, S.; Liu, W.; Liu, Y.; Liu, Z.; Chen, C. Influences of Alkyl and Aryl Substituents on Iminopyridine Fe(II)- and Co(II)-Catalyzed Isoprene Polymerization. Polymers 2016, 8, 389. https://doi.org/10.3390/polym8110389
Guo L, Jing X, Xiong S, Liu W, Liu Y, Liu Z, Chen C. Influences of Alkyl and Aryl Substituents on Iminopyridine Fe(II)- and Co(II)-Catalyzed Isoprene Polymerization. Polymers. 2016; 8(11):389. https://doi.org/10.3390/polym8110389
Chicago/Turabian StyleGuo, Lihua, Xinyu Jing, Shuoyan Xiong, Wenjing Liu, Yanlan Liu, Zhe Liu, and Changle Chen. 2016. "Influences of Alkyl and Aryl Substituents on Iminopyridine Fe(II)- and Co(II)-Catalyzed Isoprene Polymerization" Polymers 8, no. 11: 389. https://doi.org/10.3390/polym8110389
APA StyleGuo, L., Jing, X., Xiong, S., Liu, W., Liu, Y., Liu, Z., & Chen, C. (2016). Influences of Alkyl and Aryl Substituents on Iminopyridine Fe(II)- and Co(II)-Catalyzed Isoprene Polymerization. Polymers, 8(11), 389. https://doi.org/10.3390/polym8110389