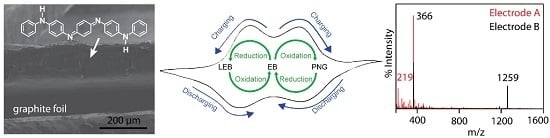
Characterization of Aniline Tetramer by MALDI TOF Mass Spectrometry upon Oxidative and Reductive Cycling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Graphene Oxide
2.2. Synthesis of Aniline Tetramer [16]
2.3. Construction of Symmetric Devices
2.4. Measurements
3. Results and Discussion
3.1. Polyaniline and Aniline Tetramer
3.2. Cycled Redox of Aniline Tetramer in Solution

3.3. Aniline Tetramer as the Active Material in Supercapacitors
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Biswas, S.; Drzal, L.T. Multi layered nanoarchitecture of graphene nanosheets and polypyrrole nanowires for high performance supercapacitor electrodes. Chem. Mater. 2010, 22, 5667–5671. [Google Scholar] [CrossRef]
- Laforgue, A.; Simon, P.; Sarrazin, C.; Fauvarque, J.F. Polythiophene-based supercapacitors. J. Power Sources 1999, 80, 142–148. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.L.; Zhao, X.S.; Wu, J.S. Graphene/polyaniline nanofiber composites as supercapacitor electrodes. Chem. Mater. 2010, 22, 1392–1401. [Google Scholar] [CrossRef]
- Shi, Y.; Peng, L.L.; Ding, Y.; Zhao, Y.; Yu, G.H. Nanostructured conductive polymers for advanced energy storage. Chem. Soc. Rev. 2015, 44, 6684–6696. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.N.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2015, 8, 702–730. [Google Scholar] [CrossRef]
- Herod, T.E.; Schlenoff, J.B. Doping-induced strain in polyaniline—Stretch electrochemistry. Chem. Mater. 1993, 5, 951–955. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, B.R.; Pan, L.J.; Yu, G.H. 3D nanostructured conductive polymer hydrogels for high-performance electrochemical devices. Energy Environ. Sci. 2013, 6, 2856–2870. [Google Scholar] [CrossRef]
- Fu, H.; Du, Z.J.; Zou, W.; Li, H.Q.; Zhang, C. Carbon nanotube reinforced polypyrrole nanowire network as a high-performance supercapacitor electrode. J. Mater. Chem. A 2013, 1, 14943–14950. [Google Scholar] [CrossRef]
- Liu, T.Y.; Finn, L.; Yu, M.H.; Wang, H.Y.; Zhai, T.; Lu, X.H.; Tong, Y.X.; Li, Y. Polyaniline and polypyrrole pseudocapacitor electrodes with excellent cycling stability. Nano Lett. 2014, 14, 2522–2527. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.L.; Yang, C.Y.; Li, X.W.; Wang, G.C. High-performance asymmetric supercapacitor based on nanoarchitectured polyaniline/graphene/carbon nanotube and activated graphene electrodes. ACS Appl. Mater. Interfaces 2013, 5, 8467–8476. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xu, Y.X.; Yao, Z.Y.; Liu, A.R.; Shi, G.Q. Supercapacitors based on flexible graphene/polyaniline nanofiber composite films. ACS Nano 2010, 4, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Kabumoto, A.; Shinozaki, K.; Watanabe, K.; Nishikawa, N. Electrochemical degradation of polyaniline. Synth. Met. 1988, 26, 349–355. [Google Scholar] [CrossRef]
- Yan, J.; Yang, L.P.; Cui, M.Q.; Wang, X.; Chee, K.; Nguyen, V.C.; Kumar, V.; Sumboja, A.; Wang, M.; Lee, P.S. Aniline tetramer-graphene oxide composites for high performance supercapacitors. Adv. Energy Mater. 2014, 4, 7. [Google Scholar] [CrossRef]
- Fusalba, F.; Gouerec, P.; Villers, D.; Belanger, D. Electrochemical characterization of polyaniline in nonaqueous electrolyte and its evaluation as electrode material for electrochemical supercapacitors. J. Electrochem. Soc. 2001, 148, A1–A6. [Google Scholar] [CrossRef]
- Santino, L.M.; Lu, Y.; Acharya, S.; Bloom, L.; Cotton, D.; Wayne, A.; D’Arcy, J.M. Enhancing cycling stability of aqueous polyaniline electrochemical capacitors. ACS Appl. Mater. Interfaces 2016, 8, 29452–29460. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tran, H.D.; Liao, L.; Duan, X.F.; Kaner, R.B. Nanoscale morphology, dimensional control, and electrical properties of oligoanilines. J. Am. Chem. Soc. 2010, 132, 10365–10373. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tran, H.D.; Kaner, R.B. Applications of oligomers for nanostructured conducting polymers. Macromol. Rapid Commun. 2011, 32, 35–49. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, J.M.; Tran, H.D.; Tung, V.C.; Tucker-Schwartz, A.K.; Wong, R.P.; Yang, Y.; Kaner, R.B. Versatile solution for growing thin films of conducting polymers. Proc. Natl. Acad. Sci. USA 2010, 107, 19673–19678. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, S.Z.; Xue, Z.J.; Guo, D. Spectroscopic and electrical characterization of some aniline oligomers and polyaniline. Synth. Met. 1986, 16, 305–315. [Google Scholar] [CrossRef]
- Epstein, A.J.; Ginder, J.M.; Zuo, F.; Bigelow, R.W.; Woo, H.S.; Tanner, D.B.; Richter, A.F.; Huang, W.S.; Macdiarmid, A.G. Insulator-to-metal transition in polyaniline. Synth. Met. 1987, 18, 303–309. [Google Scholar] [CrossRef]
- Stafstrom, S.; Bredas, J.L.; Epstein, A.J.; Woo, H.S.; Tanner, D.B.; Huang, W.S.; Macdiarmid, A.G. Polaron lattice in highly conducting polyaniline—Theoretical and optical studies. Phys. Rev. Lett. 1987, 59, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Bredas, J.L.; Street, G.B. Polarons, bipolarons, and solitons in conducting polymers. Accounts Chem. Res. 1985, 18, 309–315. [Google Scholar] [CrossRef]
- Wang, C.L.; Su, Z.B.; Martino, F. Bipolaron dynamics in nearly degenerate quasi-one-dimensional polymers. Phys. Rev. B 1986, 33, 1512–1515. [Google Scholar] [CrossRef]
- Shacklette, L.W.; Wolf, J.F.; Gould, S.; Baughman, R.H. Structure and properties of polyaniline as modeled by single-crystal oligomers. J. Chem. Phys. 1988, 88, 3955–3961. [Google Scholar] [CrossRef]
- Dolan, A.R.; Wood, T.D. Analysis of polyaniline oligomers by laser desorption ionization and solventless maldi. J. Am. Soc. Mass Spectrom. 2004, 15, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Heinze, J.; Frontana-Uribe, B.A.; Ludwigs, S. Electrochemistry of conducting polymers-persistent models and new concepts. Chem. Rev. 2010, 110, 4724–4771. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Shen, J.L.; Wang, C.Y.; Fei, H.J.; Bao, H.; Wang, G.C. All-solid-state asymmetric supercapacitor based on reduced graphene oxide/carbon nanotube and carbon fiber paper/polypyrrole electrodes. J. Mater. Chem. A 2014, 2, 1458–1464. [Google Scholar] [CrossRef]
- Wei, Y.; Hsueh, K.F.; Jang, G.W. A study of leucoemeraldine and the effect of redox reactions on the molecular-weight of chemically prepared polyaniline. Macromolecules 1994, 27, 518–525. [Google Scholar] [CrossRef]
- Arbizzani, C.; Mastragostino, M.; Meneghello, L. Polymer-based redox supercapacitors: A comparative study. Electrochim. Acta 1996, 41, 21–26. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Rudge, A.; Davey, J.; Raistrick, I.; Gottesfeld, S.; Ferraris, J.P. Conducting polymers as active materials in electrochemical capacitors. J. Power Sources 1994, 47, 89–107. [Google Scholar] [CrossRef]
- Borjas, R.; Buttry, D.A. Eqcm studies of film growth, redox cycling, and charge trapping of normal-doped and para-doped poly(thiophene). Chem. Mater. 1991, 3, 872–878. [Google Scholar] [CrossRef]
- Bryan, A.M.; Santino, L.M.; Lu, Y.; Acharya, S.; D’Arcy, J.M. Conducting polymers for pseudocapacitive energy storage. Chem. Mater. 2016, 28, 5989–5998. [Google Scholar] [CrossRef]





© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.L.; Lin, C.-W.; Shao, Y.; Chang, C.W.; Yao, F.-K.; Kowal, M.D.; Wang, H.; Yeung, M.T.; Huang, S.-C.; Kaner, R.B. Characterization of Aniline Tetramer by MALDI TOF Mass Spectrometry upon Oxidative and Reductive Cycling. Polymers 2016, 8, 401. https://doi.org/10.3390/polym8110401
Li RL, Lin C-W, Shao Y, Chang CW, Yao F-K, Kowal MD, Wang H, Yeung MT, Huang S-C, Kaner RB. Characterization of Aniline Tetramer by MALDI TOF Mass Spectrometry upon Oxidative and Reductive Cycling. Polymers. 2016; 8(11):401. https://doi.org/10.3390/polym8110401
Chicago/Turabian StyleLi, Rebecca L., Cheng-Wei Lin, Yuanlong Shao, Che Wei Chang, Fu-Kai Yao, Matthew D. Kowal, Haosen Wang, Michael T. Yeung, Shu-Chuan Huang, and Richard B. Kaner. 2016. "Characterization of Aniline Tetramer by MALDI TOF Mass Spectrometry upon Oxidative and Reductive Cycling" Polymers 8, no. 11: 401. https://doi.org/10.3390/polym8110401
APA StyleLi, R. L., Lin, C. -W., Shao, Y., Chang, C. W., Yao, F. -K., Kowal, M. D., Wang, H., Yeung, M. T., Huang, S. -C., & Kaner, R. B. (2016). Characterization of Aniline Tetramer by MALDI TOF Mass Spectrometry upon Oxidative and Reductive Cycling. Polymers, 8(11), 401. https://doi.org/10.3390/polym8110401