Dielectric Properties and Energy Storage Densities of Poly(vinylidenefluoride) Nanocomposite with Surface Hydroxylated Cube Shaped Ba0.6Sr0.4TiO3 Nanoparticles
Abstract
:1. Introduction
2. Experimental Section
3. Characterization
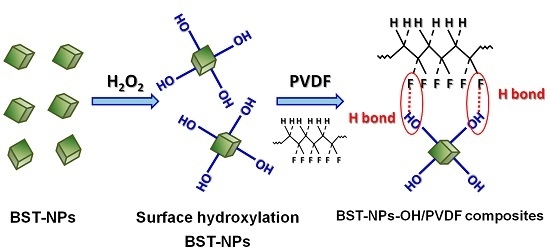
4. Results and Discussion













5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, J.H.; Huang, X.Y.; Wu, C.; Wu, X.F.; Wang, G.L; Jiang, P.K. Interfacial modification of boron nitride nanoplatelets for epoxy composites with improved thermal properties. Polymer 2012, 53, 471–480. [Google Scholar] [CrossRef]
- Liu, S.H.; Xue, S.X.; Zhang, W.Q.; Zhai, J.W.; Chen, G.H. The influence of crystalline transformation of Ba0.6Sr0.4TiO3 nanofibers/poly(vinylidene fluoride) composites on the energy storage properties by quenched technique. Ceram. Int. 2015, 41, S430–S434. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Y.; Zhang, Q.; Gu, L.; Hu, Y.; Du, J.; Lin, Y.; Nan, C.W. Ultrahigh energy density of polymer nanocomposites containing BaTiO3@TiO2 nanofibers by atomic-scale interface engineering. Adv. Mater. 2015, 27, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, X.; Gong, H.; Hao, Y.; Shen, Z.; Li, L. Core-satellite BaTiO3@SrTiO3 assemblies for a local compositionally graded relaxor ferroelectric capacitor with enhanced energy storage density and high energy efficiency. J. Mater. Chem. C 2015, 3, 750–758. [Google Scholar] [CrossRef]
- Liu, S.H.; Xue, S.X.; Shen, B.; Zhai, J.W. Reduced energy loss in poly(vinylidene fluoride) nanocomposites by filling with a small loading of core-shell structured BaTiO3/SiO2 nanofibers. Appl. Phys. Lett. 2015, 107, 032907. [Google Scholar]
- Wang, G.; Huang, X.; Jiang, P. Increasing the energy efficiency and breakdown strength of high-energy-density polymer nanocomposites by engineering the Ba0.7Sr0.3TiO3 nanowire surface via reversible addition—fragmentation chain transfer polymerization. J. Phys. Chem. C 2015, 7, 18017–18027. [Google Scholar]
- Shen, Y.; Hu, Y.; Chen, W.; Wang, J.; Guan, Y.; Du, J.; Zhang, X.; Ma, J.; Li, M.; Lin, Y. Modulation of topological structure induces ultrahigh energy density of graphene/Ba0.6Sr0.4TiO3 nanofiber/polymer nanocomposites. Nano Energy 2015, 18, 176–186. [Google Scholar] [CrossRef]
- Liu, S.H.; Xiao, S.; Xiu, S.M.; Shen, B.; Zhai, J.W.; An, Z. Poly(vinylidene fluoride) nanocomposite capacitors with a significantly enhanced dielectric constant and energy density by filling with surface-fluorinated Ba0.6Sr0.4TiO3 nanofibers. RSC Adv. 2015, 5, 40692–40699. [Google Scholar] [CrossRef]
- Tang, H.; Zhou, Z.; Bowland, C.C.; Sodano, H.A. Synthesis of calcium copper titanate (CaCu3Ti4O12) nanowires with insulating SiO2 barrier for low loss high dielectric constant nanocomposites. Nano Energy 2015, 17, 302–307. [Google Scholar] [CrossRef]
- Chu, B.J.; Zhou, X.; Ren, K.L.; Neese, B.; Lin, M.R.; Wang, Q.; Bauer, F.; Zhang, Q.M. A dielectric polymer with high electric energy density and fast discharge speed. Science 2006, 313, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Zhai, J.W. Improving the dielectric constant and energy density of poly(vinylidene fluoride) composites induced by surface-modified SrTiO3 nanofibers by polyvinylpyrrolidone. J. Mater. Chem. A 2015, 3, 1511–1517. [Google Scholar] [CrossRef]
- Dang, Z.M.; Yuan, J.K.; Yao, S.H.; Liao, R.J. Preparation and dielectric properties of core–shell structured Ag@polydopamine/poly(vinylidene fluoride) composites. Adv. Mater. 2013, 25, 6334–6365. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jiang, P. Core–shell structured high-k polymer nanocomposites for energy storage and dielectric applications. Adv. Mater. 2015, 27, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Zhai, J.W.; Wang, J.W.; Xue, S.X.; Zhang, W.Q. Enhanced energy storage density in poly(vinylidene fluoride) nanocomposites by a small loading of suface-hydroxylated Ba0.6Sr0.4TiO3 nanofibers. ACS. Appl Mater. Inter. 2014, 6, 1533–1540. [Google Scholar]
- Tang, H.X.; Zhou, Z.; Sodano, H.A. Relationship between BaTiO3 nanowire aspect ratio and the dielectric permittivity of nanocomposites. ACS. Appl Mater. Interfaces 2014, 6, 5450–5455. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.M.; Yuan, J.K.; Zha, J.W.; Zhou, T.; Li, S.T.; Hu, G.H. Fundamentals, processes and applications of high-permittivity polymer–matrix composites. Prog. Mater. Sci. 2012, 57, 660–723. [Google Scholar] [CrossRef]
- Luo, B.; Wang, X.; Wang, Y.; Li, L. Fabrication, characterization, properties and theoretical analysis of ceramic/PVDF composite flexible films with high dielectric constant and low dielectric loss. J. Mater. Chem A 2014, 2, 510–519. [Google Scholar] [CrossRef]
- Liu, S.H.; Xue, S.X.; Zhang, W.Q.; Zhai, J.W.; Chen, G.H. Significantly enhanced dielectric property in PVDF nanocomposites flexible films through a small loading of surface-hydroxylated Ba0.6Sr0.4TiO3 nanotubes. J. Mater. Chem A 2014, 2, 18040–18046. [Google Scholar] [CrossRef]
- Song, Y.; Shen, Y.; Hu, P.H.; Lin, Y.H.; Li, M.; Nan, C.W. Significant enhancement in energy density of polymer composites induced by dopamine-modified Ba0.6Sr0.4TiO3 nanofibers. Appl Phys. Lett 2012, 101. [Google Scholar] [CrossRef]
- Liu, S.H.; Zhai, J.W. A small loading of surface-modified Ba0.6Sr0.4TiO3 nanofiber-filled nanocomposites with enhanced dielectric constant and energy density. RSC. Adv. 2014, 4, 40973–40979. [Google Scholar] [CrossRef]
- Xie, L.Y.; Huang, X.Y.; Wu, C.; Jiang, P.K. Core-shell structured poly(methyl methacrylate)/BaTiO3 nanocomposites prepared by in situ atom transfer radical polymerization: A route to high dielectric constant materials with the inherent low loss of the base polymer. J. Mater. Chem 2011, 21, 5897–5906. [Google Scholar] [CrossRef]
- Xie, L.Y.; Huang, X.Y.; Huang, Y.H.; Yang, K.; Jiang, P.K. Core-shell structured hyperbranched aromatic polyamide/BaTiO3 Hybrid filler for poly(vinylidene fluoride-trifluoroethylene-chlorofluoroethylene) nanocomposites with the dielectric constant comparable to that of percolative composites. ACS Appl. Mater. Interfaces 2013, 5, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.Y.; Huang, X.Y.; Yang, K.; Li, S.T.; Jiang, P.K. “Grafting to” route to PVDF-HFP-GMA/BaTiO3 nanocomposites with high dielectric constant and high thermal conductivity for energy storage and thermal management applications. J. Mater. Chem A 2014, 2, 5244–5251. [Google Scholar] [CrossRef]
- Kim, P.; Doss, N.M.; Tillotson, J.P.; Hotchkiss, P.J.; Pan, M.J.; Marder, S.R.; Li, J.Y.; Calame, J.P.; Perry, J.W. High energy density nanocomposites based on surface-modified BaTiO3 and a ferroelectric polymer. ACS Nano 2009, 3, 2581–2592. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Niu, Y.J.; Zhou, Y.C.; Bai, Y.Y.; Wang, H. Nanocomposites of surface-modified BaTiO3 nanoparticles filled ferroelectric polymer with enhanced energy density. J. Am. Ceram. Soc. 2013, 96, 2519–2524. [Google Scholar] [CrossRef]
- Wang, Z.P.; Nelson, J.K.; Hillborg, H.; Zhao, S.; Schadler, L.S. Dielectric constant and breakdown strength of polymer composites with high aspect ratio fillers studied by finite element models. Compos. Sci. Technol. 2013, 76, 29–36. [Google Scholar] [CrossRef]
- Hu, G.; Gao, F.; Kong, J.; Yang, S.; Zhang, Q.; Liu, Z.; Zhang, Y.; Sun, H. Preparation and dielectric properties of poly(vinylidene fluoride)/Ba0.6Sr0.4TiO3 composites. J. Alloy. Compd. 2015, 619, 686–692. [Google Scholar] [CrossRef]
- Thomas, P.; Varughese, K.; Dwarakanath, K.; Varma, K. Dielectric properties of poly(vinylidene fluoride)/CaCu3Ti4O12 composites. Compos. Sci. Technol. 2010, 70, 539–545. [Google Scholar] [CrossRef]
- Yang, K.; Huang, X.Y.; Huang, Y.H.; Xie, L.Y.; Jiang, P.K. Fluoro-polymer@BaTiO3 hybrid nanoparticles prepared via RAFT polymerization: Toward ferroelectric polymer nanocomposites with high dielectric constant and low dielectric loss for energy storage application. Chem. Mater. 2013, 25, 2327–2338. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, J.; Yuan, Q.; Niu, Y.; Bai, Y.; Wang, H. Significantly enhanced breakdown strength and energy density in sandwich-structured barium titanate/poly(vinylidene fluoride) Nanocomposites. Adv. Mater. 2015, 27, 6658–6663. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Xiu, S.; Shen, B.; Zhai, J.; Kong, L.B. Dielectric Properties and Energy Storage Densities of Poly(vinylidenefluoride) Nanocomposite with Surface Hydroxylated Cube Shaped Ba0.6Sr0.4TiO3 Nanoparticles. Polymers 2016, 8, 45. https://doi.org/10.3390/polym8020045
Liu S, Xiu S, Shen B, Zhai J, Kong LB. Dielectric Properties and Energy Storage Densities of Poly(vinylidenefluoride) Nanocomposite with Surface Hydroxylated Cube Shaped Ba0.6Sr0.4TiO3 Nanoparticles. Polymers. 2016; 8(2):45. https://doi.org/10.3390/polym8020045
Chicago/Turabian StyleLiu, Shaohui, Shaomei Xiu, Bo Shen, Jiwei Zhai, and Ling Bing Kong. 2016. "Dielectric Properties and Energy Storage Densities of Poly(vinylidenefluoride) Nanocomposite with Surface Hydroxylated Cube Shaped Ba0.6Sr0.4TiO3 Nanoparticles" Polymers 8, no. 2: 45. https://doi.org/10.3390/polym8020045
APA StyleLiu, S., Xiu, S., Shen, B., Zhai, J., & Kong, L. B. (2016). Dielectric Properties and Energy Storage Densities of Poly(vinylidenefluoride) Nanocomposite with Surface Hydroxylated Cube Shaped Ba0.6Sr0.4TiO3 Nanoparticles. Polymers, 8(2), 45. https://doi.org/10.3390/polym8020045