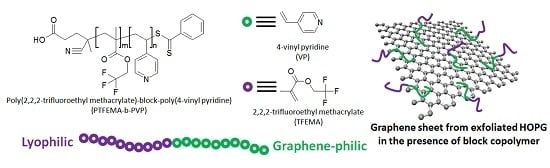
Amphiphilic Fluorinated Block Copolymer Synthesized by RAFT Polymerization for Graphene Dispersions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of Poly(2,2,2-trifluoroethyl methacrylate) (PTFEMA) Macro-Reversible Addition-Fragmentation Chain Transfer (RAFT) Agents
2.3. Synthesis of Poly(2,2,2-trifluoroethyl methacrylate)-block-poly(4-vinyl pyridine) (PTFEMA-b-PVP) Block Copolymers
2.4. Preparation of Graphene Dispersions
2.5. Adhesion Force Study Using Modified Atomic Force Microscope (AFM) Cantilever
2.6. Characterization
3. Results and Discussion
3.1. PTFEMA-b-PVP Block Copolymers
3.2. Adhesion Force Analyses
3.3. Stability of Graphene Dispersions
3.4. Graphene Nanoplatelets
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Moore, A.L.; Zhu, Y.; Li, X.; Chen, S.; Shi, L.; Ruoff, R.S. Thermal transport in suspended and supported monolayer graphene grown by chemical vapor deposition. Nano Lett. 2010, 10, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Faugeras, C.; Faugeras, B.; Orlita, M.; Potemski, M.; Nair, R.R.; Geim, A.K. Thermal conductivity of graphene in corbino membrane geometry. ACS Nano 2010, 4, 1889–1892. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Macosko, C.W. Processing-property relationships of polycarbonate/graphene composites. Polymer 2009, 50, 3797–3809. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Q.; Huang, Y.; Ma, Y.; Yin, S.; Zhang, X.; Sun, W.; Chen, Y. Organic photovoltaic devices based on a novel acceptor material: Graphene. Adv. Mater. 2008, 20, 3924–3930. [Google Scholar] [CrossRef]
- Huang, L.; Huang, Y.; Liang, J.; Wan, X.; Chen, Y. Graphene-based conducting inks for direct inkjet printing of flexible conductive patterns and their applications in electric circuits and chemical sensors. Nano Res. 2011, 4, 675–684. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, L.; Mullen, K. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.-Y.; Hong, J.-Y.; Jang, J. Flexible and transparent graphene films as acoustic actuator electrodes using inkjet printing. Chem. Commun. 2011, 47, 8527–8529. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 50, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Obraztsov, A.N. Chemical vapour deposition: Making graphene on a large scale. Nat. Nanotechnol. 2009, 4, 212–213. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.M.; Kim, H.; Lee, J.H.; Song, Y.-W. Multilayered graphene efficiently formed by mechanical exfoliation for nonlinear saturable absorbers in fiber mode-locked lasers. Appl. Phys. Lett. 2010, 97, 211102. [Google Scholar] [CrossRef]
- Lin, T.; Chen, J.; Bi, H.; Wan, D.; Huang, F.; Xie, X.; Jiang, M. Facile and economical exfoliation of graphite for mass production of high-quality graphene sheets. J. Mater. Chem. A 2013, 1, 500–504. [Google Scholar] [CrossRef]
- Han, P.; Akagi, K.; Canova, F.F.; Mutoh, H.; Shiraki, S.; Lwaya, K.; Weiss, P.S.; Asao, N.; Hitosugi, T. Bottom-up graphene-nanoribbon fabrication reveals chiral edges and enantioselectivity. ASC Nano 2014, 8, 9181–9187. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Akagi, K.; Canova, F.F.; Shimizu, R.; Oguchi, H.; Shiraki, S.; Weiss, P.S.; Asao, N.; Hitosugi, T. Self-assembly strategy for fabricating connected graphene nanoribbons. ACS Nano 2015, 9, 12035–12044. [Google Scholar] [CrossRef] [PubMed]
- Norimatsu, W.; Kusunoki, M. Epitaxial graphene on SiC{0001}: Advances and perspectives. Phys. Chem. Chem. Phys. 2014, 16, 3501–3511. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, A.; Samori, P. Graphene via sonication assisted liquid-phase exfoliation. Chem. Soc. Rev. 2014, 43, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhang, C.; Hao, R.; Hou, Y. Liquid-phase exfoliation, functionalization and applications of graphene. Nanoscale 2011, 3, 2118–2126. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Müller, M.B.; Jilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar]
- Wojtoniszak, M.; Chen, X.; Kalenczuk, R.J.; Wajda, A.; Łapczuk, J.; Kurzewski, M.; Drozkzik, M.; Chu, P.K.; Borowiak-Palen, E. Synthesis, dispersion, and cytocompatibility of graphene oxide and reduced graphene oxide. Colloids Surf. B Biointerfaces 2012, 89, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; de, S.; Wang, Z.; McGovern, I.T.; et al. Liquid phase production of graphene by exfoliation of graphite in surfactant/water solution. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef] [PubMed]
- Lotya, M.; King, P.; Khan, U.; De, S.; Coleman, J.N. High-concentration, surfactant-stablized graphene dispersion. ACS Nano 2010, 4, 3155–3162. [Google Scholar] [CrossRef] [PubMed]
- Vadukumpully, S.; Paul, J.; Valiyaveettil, S. Cationic surfactant mediated exfoliation of graphite into graphene flakes. Carbon 2009, 47, 3288–3294. [Google Scholar] [CrossRef]
- Hamilton, C.E.; Lomeda, J.R.; Sun, Z.; Tour, J.M.; Barron, A.R. High-yield organic dispersions of unfunctionalized graphene. Nano Lett. 2009, 9, 3460–3462. [Google Scholar] [CrossRef] [PubMed]
- Nuvoli, D.; Valentini, L.; Alzari, V.; Scognamillo, S.; Bon, S.B.; Piccinini, M.; Illescas, J.; Mariani, A. High concentration few-layer graphene sheets obtained by liquid phase exfoliation of graphite in ionic liquid. J. Mater. Chem. 2011, 21, 3428–3431. [Google Scholar] [CrossRef]
- O’Neill, A.; Khan, U.; Nirmalraj, P.N.; Boland, J.; Coleman, J.N. Graphene dispersion and exfoliation in low boiling point solvents. J. Phys. Chem. C 2011, 115, 5422–5428. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Georgakilas, V.; Zboril, R.; Steriotis, T.A.; Stubos, A.K. Liquid-phase exfoliation of graphite towards solubilized graphenes. Small 2009, 5, 1841–1845. [Google Scholar] [CrossRef] [PubMed]
- Perumal, S.; Park, K.T.; Lee, H.M.; Cheong, I.W. PVP-b-PEO block copolymers for stable aqueous and ethanolic graphene dispersions. J. Colloid Interface Sci. 2016, 464, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Zu, S.-Z.; Han, B.-H. Aqueous dispersion of graphene sheets stabilized by pluronic copolymers: Formation of supramolecular hydrogel. J. Phys. Chem. C 2009, 113, 13651–13657. [Google Scholar] [CrossRef]
- Popescu, M.-T.; Tasis, D.; Papadimitriou, K.D.; Gkermpoura, S.; Galiotis, C.; Tsitsilianis, C. Colloidal stabilization of graphene sheets by ionizable amphiphilic block copolymers in various media. RSC Adv. 2015, 5, 89447–89460. [Google Scholar] [CrossRef]
- Voloshina, E.N.; Mollenhauer, D.; Chiappisi, L.; Paulus, B. Theoretical study on the adsorption of pyridine derivatives on graphene. Chem. Phys. Lett. 2011, 510, 220–223. [Google Scholar] [CrossRef]
- Wisniewsk, M.; Szewezuk-Karpisz, K. Removal possibilities of colloidal chromium (III) oxide from water using polyacrylic acid. Environ. Sci. Pollut. Res. 2013, 20, 3657–3669. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Zhu, L.; Ye, S.; Jo, S.-B.; Oh, W.-C. Photocatalytic and reusability studies of novel ZnSe/graphene nanocomposites synthesized via one pot hydrothermal techniques. Asian J. Chem. 2014, 26, 4097–4102. [Google Scholar]
- We, W.; Yu, Q.; Peng, P.; Liu, Z.; Bao, J.; Pei, S.-S. Control of thinkness uniformity and grain size in graphene films for transparent conductive electrodes. Nanotechnology 2012, 23, 035603. [Google Scholar]
- Gayathri, S.; Jayabal, P.; Kottaisamy, M.; Ramakrishnan, V. Synthesis of few layer graphene by direct exfoliation of graphite and a Raman spectroscopic study. AIP Adv. 2014, 4, 027116. [Google Scholar] [CrossRef]
- Das, A.; Chakraborty, B.; Sood, A.K. Raman spectroscopy of graphene on different substrates and influence of defects. Bull. Mater. Sci. 2008, 31, 579–584. [Google Scholar] [CrossRef]








| Designation | Mn for PTFEMA 1 (g/mol) | Mn for PVP 2 (g/mol) | Degree of polymerization | Graphene concentration 3 (mg/mL) |
|---|---|---|---|---|
| 1 | 11,092 | 4,338 | PTFEMA66-b-PVP41 | 0.260 |
| 2 | 11,092 | 21,581 | PTFEMA66-b-PVP205 | 0.350 |
| 3 | 22,870 | 3,309 | PTFEMA136-b-PVP31 | 0.275 |
| 4 | 22,870 | 20,410 | PTFEMA136-b-PVP194 | 0.385 |
| Designation | I2D/IG | ID/IG |
|---|---|---|
| Monolayer graphene | >2.0 | – |
| Graphite | <0.4 | – |
| Graphene oxide | – | >0.9 |
| M-25, raw | 0.4281 | 0.1474 |
| 1 | 0.5601 | 0.2250 |
| 2 | 0.6125 | 0.1707 |
| 3 | 0.4692 | 0.2625 |
| 4 | 0.4983 | 0.2307 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.M.; Perumal, S.; Cheong, I.W. Amphiphilic Fluorinated Block Copolymer Synthesized by RAFT Polymerization for Graphene Dispersions. Polymers 2016, 8, 101. https://doi.org/10.3390/polym8030101
Lee HM, Perumal S, Cheong IW. Amphiphilic Fluorinated Block Copolymer Synthesized by RAFT Polymerization for Graphene Dispersions. Polymers. 2016; 8(3):101. https://doi.org/10.3390/polym8030101
Chicago/Turabian StyleLee, Hyang Moo, Suguna Perumal, and In Woo Cheong. 2016. "Amphiphilic Fluorinated Block Copolymer Synthesized by RAFT Polymerization for Graphene Dispersions" Polymers 8, no. 3: 101. https://doi.org/10.3390/polym8030101
APA StyleLee, H. M., Perumal, S., & Cheong, I. W. (2016). Amphiphilic Fluorinated Block Copolymer Synthesized by RAFT Polymerization for Graphene Dispersions. Polymers, 8(3), 101. https://doi.org/10.3390/polym8030101