A Heterobimetallic Anionic 3,6-Connected 2D Coordination Polymer Based on Nitranilate as Ligand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Precursor Salt Na3[Fe(C6N2O8)3]
2.2. Synthesis of [(DAMS)2{FeNa(C6N2O8)3}·CH3CN]n (1)
2.3. Single Crystal X-ray Structure Determination
2.4. Physical Measurements
3. Results
3.1. Synthesis of Compound [(DAMS)2{FeNa(C6N2O8)3}·CH3CN]n (1)
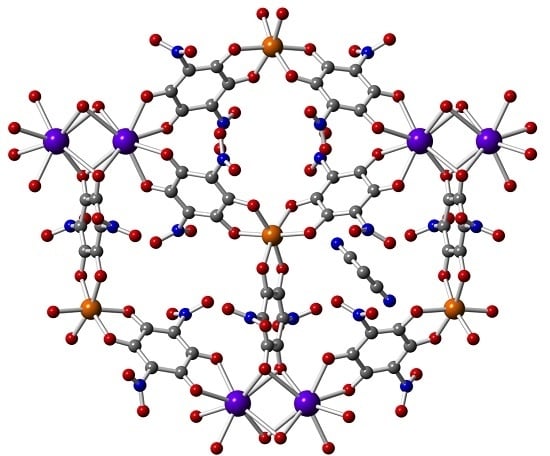
3.2. Crystal Structure of Compound [(DAMS)2{FeNa(C6N2O8)3}·CH3CN]n (1)
3.3. Magnetic Properties of Compound [(DAMS)2{FeNa(C6N2O8)3}·CH3CN]n (1)
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Farha, O.K.; Hupp, J.T. Rational design, synthesis, purification, and activation of metal–organic framework materials. Acc. Chem. Res. 2010, 43, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.R.; Zheng, Y.; Stang, P.J. Metal–organic frameworks and self-assembled supramolecular coordination complexes: Comparing and contrasting the design, synthesis and functionality of metal-organic materials. Chem. Rev. 2012, 113, 734–777. [Google Scholar] [CrossRef] [PubMed]
- Leininger, S.; Olenyuk, B.; Stang, P.J. Self-assembly of discrete cyclic nanostructures mediated by transition metals. Chem. Rev. 2000, 100, 853–908. [Google Scholar] [CrossRef] [PubMed]
- Pettinari, C.; Tabacaru, A.; Galli, S. Coordination polymers and metal–organic frameworks based on poly(pyrazole)-containing ligands. Coord. Chem. Rev. 2016, 307, 1–31. [Google Scholar] [CrossRef]
- Li, B.; Chrzanowski, M.; Zhang, Y.; Ma, S. Applications of metal–organic frameworks featuring multi-functional sites. Coord. Chem. Rev. 2016, 307, 106–129. [Google Scholar] [CrossRef]
- Seoane, B.; Castellanos, S.; Dikhtiarenko, A.; Kapteijn, F.; Gascon, J. Multi-scale crystal engineering of metal organic frameworks. Coord. Chem. Rev. 2016, 307, 147–187. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.Ö.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; Yaghi, O.M. Ultrahigh porosity in metal–organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yang, Z.; Bai, J.; Zheng, B.; Li, Y.; Li, S. Highly selective CO2 capture of an agw-type metal–organic framework with inserted amides: Experimental and theoretical studies. Chem. Commun. 2012, 48, 3058–3060. [Google Scholar] [CrossRef] [PubMed]
- Brozek, C.K.; Dinca, M. Cation exchange at the secondary building units of metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 5456–5467. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lin, W. Metal–organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014, 43, 5982–5993. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Xue, M.; Zhu, G. Metal–organic framework membranes: From synthesis to separation application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef] [PubMed]
- Givaja, G.; Amo-Ochoa, P.; Gómez-García, C.J.; Zamora, F. Electrical conductive coordination polymers. Chem. Soc. Rev. 2012, 41, 115–147. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Sadakiyo, M.; Shigematsu, A.; Kitagawa, H. Proton-conductive metal–organic frameworks. Bull. Chem. Soc. Jpn. 2016, 89, 1–10. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal–organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Stavila, V.; Talin, A.A.; Allendorf, M.D. MOF-based electronic and opto-electronic devices. Chem. Soc. Rev. 2014, 43, 5994–6010. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, J.; Song, C.; Chen, T.; Sun, Z.; Wang, S.; Luo, J.; Hong, M. A 3D polar nanotubular coordination polymer with dynamic structural transformation and ferroelectric and nonlinear-optical properties. Inorg. Chem. 2012, 51, 2438–2442. [Google Scholar] [CrossRef] [PubMed]
- Batten, S.R.; Murray, K.S. Structure and magnetism of coordination polymers containing dicyanamide and tricyanomethanide. Coord. Chem. Rev. 2003, 246, 103–130. [Google Scholar] [CrossRef]
- Evans, O.R.; Lin, W. Crystal engineering of NLO materials based on metal–organic coordination networks. Acc. Chem. Res. 2002, 35, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Galan-Mascaros, J.R.; Gómez-García, C.J.; Laukhin, V. Coexistence of ferromagnetism and metallic conductivity in a molecule-based layered compound. Nature 2000, 408, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R. Supramolecular synthons in crystal engineering: A new organic synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Brammer, L. Hydrogen bonds in inorganic chemistry: Application to crystal design in crystal design: Structure and function. In Perspectives in Supramolecular Chemistry; Desiraju, G.R., Ed.; Wiley: Chichester, UK, 2003; Volume 7, pp. 1–76. [Google Scholar]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’keeffe, M.; Yaghi, O.M. Modular chemistry: Secondary building units as a basis for the design of highly porous and robust metal–organic carboxylate frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Tranchemontagne, D.J.; Mendoza-Cortés, J.L.; O’Keeffe, M.; Yaghi, O.M. Secondary building units, nets and bonding in the chemistry of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1257–1283. [Google Scholar] [CrossRef] [PubMed]
- Das, L.K.; Gómez-García, C.J.; Drew, M.G. B.; Ghosh, A. Playing with different metalloligands [NiL] and Hg to [NiL] ratios to tune the nuclearity of Ni(II)–Hg(II) complexes: Formation of Di-, Tri-, Hexa- and nona-nuclear Ni–Hg clusters. Polyhedron 2015, 87, 311–320. [Google Scholar] [CrossRef]
- Biswas, S.; Naiya, S.; Gómez-García, C.J.; Ghosh, A. Synthesis of the first heterometalic star-shaped oxido-bridged MnCu3 complex and its conversion into trinuclear species modulated by pseudohalides (N3−, NCS− and NCO−): Structural analyses and magnetic properties. Dalton Trans. 2012, 41, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Verdaguer, M.; Kahn, O.; Sletten, J.; Renard, J.P. Magnetism of manganese(II)copper(II) and nickel(II)copper(II) ordered bimetallic chains. Crystal structure of MnCu(Pba)(H2O)3.2H2O (Pba = 1,3-propylenebis(oxamato)). Inorg. Chem. 1987, 26, 138–143. [Google Scholar] [CrossRef]
- Atzori, M.; Benmansour, S.; Mínguez Espallargas, G.; Clemente-León, M.; Abhervé, A.; Gómez-Claramunt, P.; Coronado, E.; Artizzu, F.; Sessini, E.; Deplano, P.; et al. A family of layered chiral porous magnets exhibiting tunable ordering temperatures. Inorg. Chem. 2013, 52, 10031–10040. [Google Scholar] [CrossRef] [PubMed]
- Benmansour, S.; Vallés-García, C.; Gómez-Claramunt, P.; Mínguez Espallargas, G.; Gómez-García, C.J. 2D and 3D anilato-based heterometallic M(I)M(III) lattices: The missing link. Inorg. Chem. 2015, 54, 5410–5418. [Google Scholar] [CrossRef] [PubMed]
- Benmansour, S.; Gómez-Claramunt, P.; Vallés-García, C.; Espallargas, G.M.; Gómez-García, C.J. Key role of the cation in the crystallization of chiral tris(anilato)metalate magnetic anions. Cryst. Growth Des. 2016, 16, 518–526. [Google Scholar] [CrossRef]
- Martin, L.; Day, P.; Nakatsuji, S.; Yamada, J.; Akutsu, H.; Horton, P. A molecular charge transfer salt of BEDT-TTF containing a single enantiomer of tris(oxalato)chromate(III) crystallized from a chiral solvent. CrystEngComm 2010, 12, 1369–1372. [Google Scholar] [CrossRef]
- Martin, L.; Day, P.; Clegg, W.; Harrington, R.W.; Horton, P.N.; Bingham, A.; Hursthouse, M.B.; McMillan, P.; Firth, S. Multi-layered molecular charge-transfer salts containing alkali metal ions. J. Mater. Chem. 2007, 17, 3324–3329. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, H.; Twamley, B.; Shreeve, J.M. Highly dense nitranilates-containing nitrogen-rich cations. Chem. Eur. J. 2009, 15, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G. G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic corrections and Pascal’s constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Benmansour, S.; Setifi, F.; Triki, S.; Gómez-García, C.J. Linkage isomerism in coordination polymers. Inorg. Chem. 2012, 51, 2359–2365. [Google Scholar] [CrossRef] [PubMed]
- Robl, C.; Weiss, A. Complexes with substituted 2,5-dihydroxy-para-benzochinones Zn(C6(NO2)2O4).2H2O. Z. Naturforsch. 1986, 41, 1337–1340. [Google Scholar]
- Bénard, S.; Yu, P.; Audière, J.P.; Rivière, E.; Clément, R.; Guilhem, J.; Tchertanov, L.; Nakatani, K. Structure and NLO properties of layered bimetallic oxalato-bridged ferromagnetic networks containing stilbazolium-shaped chromophores. J. Am. Chem. Soc. 2000, 122, 9444–9454. [Google Scholar] [CrossRef]
- Groom, C.R.; Allen, F.H. The cambridge structural database in retrospect and prospect. Angew. Chem. Int. Ed. 2014, 53, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Lah, M.S.; Gibney, B.R.; Tierney, D.L.; Penner-Hahn, J.E.; Pecoraro, V.L. The fused metallacrown anion Na2[Na0.5[Ga(salicylhydroximate)]4]2(μ2-OH)4 is an inorganic analog of a cryptate. J. Am. Chem. Soc. 1993, 115, 5857–5858. [Google Scholar] [CrossRef]
- De Zorzi, R.; Guidolin, N.; Randaccio, L.; Geremia, S. A bifunctionalized porous material containing discrete assemblies of copper–porphyrins and calixarenes metallated by ion diffusion. CrystEngComm 2010, 12, 4056–4058. [Google Scholar] [CrossRef]
- Zorzi, R.D.; Guidolin, N.; Randaccio, L.; Purrello, R.; Geremia, S. Nanoporous crystals of calixarene/porphyrin supramolecular complex functionalized by diffusion and coordination of metal ions. J. Am. Chem. Soc. 2009, 131, 2487–2489. [Google Scholar] [CrossRef] [PubMed]
- Mengle, K.; Longenecker, E.; Zeller, M.; Zaleski, C. One-dimensional coordination polymers of 12-metallacrown-4 complexes: {Na2(L)212-MCMnIII(N)Shi-4]}n, where L is either –O2CCH2CH3 or –O2CCH2CH2CH3. J. Chem. Cryst. 2015, 45, 36–43. [Google Scholar] [CrossRef]
- Azar, M.R.; Boron, T.T.; Lutter, J.C.; Daly, C.I.; Zegalia, K.A.; Nimthong, R.; Ferrence, G.M.; Zeller, M.; Kampf, J.W.; Pecoraro, V.L.; et al. Controllable Formation of heterotrimetallic coordination compounds: Systematically incorporating lanthanide and alkali metal ions into the manganese 12-metallacrown-4 framework. Inorg. Chem. 2014, 53, 1729–1742. [Google Scholar] [CrossRef] [PubMed]
- Daly, C.I.; Zeller, M.; Zaleski, C.M. Crystal structure of di-μ-chloro-acetato-hexakis (dimethylformamide)-tetrakis-(μ-N,2-dioxido-benzene-1-carboximidato)tetra-manganese(III)disodium dimethyl-formamide disolvate. Acta Cryst. E 2014, 70, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Gibney, B.R.; Wang, H.; Kampf, J.W.; Pecoraro, V.L. Structural evaluation and solution integrity of alkali metal salt complexes of the manganese 12-metallacrown-4(12-MC-4) structural type. Inorg. Chem. 1996, 35, 6184–6193. [Google Scholar] [CrossRef]
- Kessissoglou, D.P.; Bodwin, J.J.; Kampf, J.; Dendrinou-Samara, C.; Pecoraro, V.L. Pseudohalide complexation by manganese 12-metallacrowns-4 complexes. Inorg. Chim. Acta 2002, 331, 73–80. [Google Scholar] [CrossRef]
- Klapotke, T.M.; Sproll, S.M. Synthesis and investigation of 1,2,3,4-thiatriazol-5-ylcarbamates. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 1803–1813. [Google Scholar] [CrossRef]
- Mizutani, M.; Miwa, S.; Fukushima, N.; Funahashi, Y.; Ozawa, T.; Jitsukawa, K.; Masuda, H. Syntheses and structures of tetrakis(1-methyluracilato)palladium complexes capturing alkali metal ions. A new type of metallo-podand. Inorg. Chim. Acta 2002, 339, 543–550. [Google Scholar] [CrossRef]
- Li, Y.; Martell, A.E.; Hancock, R.D.; Reibenspies, J.H.; Anderson, C.J.; Welch, M.J. N,N′-Ethylenedi-l-cysteine (EC) and its metal complexes: Synthesis, characterization, crystal structures, and equilibrium constants. Inorg. Chem. 1996, 35, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Chung, T. Oxalic acid complexes: promising draw solutes for forward osmosis (FO) in protein enrichment. Chem. Commun. 2015, 51, 4854–4857. [Google Scholar] [CrossRef] [PubMed]
- Palkina, K.K.; Kochetov, A.N.; Churakov, A.V.; Sergienko, V.S. Synthesis and crystal structure of the sodium complex with 2-(diphenylacetyl)indandione-1,3. Russ. J. Inorg. Chem. 2011, 56, 1258–1263. [Google Scholar] [CrossRef]
- Coelho, A.C.; Almeida Paz, F.A.; Klinowski, J.; Pillinger, M.; Gonçalves, I.S. Synthesis and structure of a sodium complex of an aromatic β-diketone and pyrazolylpyridine. Molecules 2006, 11, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, L.; Song, F.; Wang, B.; Yuan, H.; Ye, C. Structure and NMR spectroscopy in solid state and solution of Na2[μ2-(C6H4O2)2](C6H4OOH)24−. Chin. J. Chem. 2006, 24, 336–340. [Google Scholar] [CrossRef]
- Bock, H.; Nick, S.; Näther, C.; Bats, J.W. Strukturen ladungsgestörter moleküle, 47 dinatrium-und dikalium-nitranilate: Die cyanin-verzerrung der kohlenstoff-sechsringe. Z. Naturforsch. B Chem. Sci. 1994, 49, 1021–1030. [Google Scholar] [CrossRef]
- Cariati, E.; Ugo, R.; Cariati, F.; Roberto, D.; Masciocchi, N.; Galli, S.; Sironi, A. J-aggregates granting giant second-order NLO responses in self-assembled hybrid inorganic–organic materials. Adv. Mater. 2001, 13, 1665–1668. [Google Scholar] [CrossRef]
- O’Connor, C.J. Magnetochemistry-advances in theory and experimentation. Prog. Inorg. Chem. 1982, 29, 203–283. [Google Scholar]
- Boca, R. Zero-field splitting in metal complexes. Coord. Chem. Rev. 2004, 248, 757–815. [Google Scholar] [CrossRef]





| Compound | 1 |
|---|---|
| Formula | C52H41FeN11NaO24 |
| F. Wt. | 1,282.80 |
| Crystal system | Orthorhombic |
| Space group | Ccca |
| a (Å) | 17.0607(8) |
| b (Å) | 24.6580(12) |
| c (Å) | 26.2191(14) |
| α (°) | 90 |
| β (°) | 90 |
| γ (°) | 90 |
| V (Å3) | 11,029.9(10) |
| Z | 8 |
| T (K) | 120 |
| (g.cm−3) | 1.545 |
| μ (cm−1) | 0.379 |
| F(000) | 5272 |
| Crystal size (mm3) | 0.12 × 0.09 × 0.05 |
| θ range (°) | 2.86–25.06 |
| Total reflections | 39,529 |
| Unique reflections | 4,885 |
| Rint | 0.1194 |
| Data with I > 2σ(I) | 2,904 |
| Nv | 425 |
| a R1 | 0.0613 |
| b wR2 | 0.1214 |
| c GooF | 1.057 |
| Δ (eÅ−3) | +0.626 |
| Δ (eÅ−3) | −0.386 |
| Atoms | Distance | Atoms | Distance |
| Fe1–O2 | 1.995(2) | Na1–O1 c | 2.876(3) |
| Fe1–O2 a | 1.995(2) | Na1–O1 d | 2.876(3) |
| Fe1–O3 | 2.016(2) | Na1–O5 | 2.466(3) |
| Fe1–O3 a | 2.016(2) | Na1–O5 b | 2.466(3) |
| Fe1–O12 | 2.016(2) | Na1–O6 | 2.417(3) |
| Fe1–O12 a | 2.016(2) | Na1–O6 b | 2.417(3) |
| Na1–O1 | 2.428(3) | Na1–Na1 d | 3.256(4) |
| Na1–O1 b | 2.428(3) | ||
| Atoms | Angle | Atoms | Angle |
| O2–Fe1–O2 a | 172.77(14) | O3–Fe1–O12 | 166.86(10) |
| O2–Fe1–O3 | 79.74(10) | O3 a–Fe1–O12 | 95.48(10) |
| O2 a–Fe1–O3 | 95.18(10) | O2–Fe1–O12 a | 96.89(10) |
| O2–Fe1–O3 a | 95.18(10) | O2 a–Fe1–O12 a | 88.67(10) |
| O2 a–Fe1–O3 a | 79.71(10) | O3–Fe1–O12 a | 95.48(10) |
| O3–Fe1–O3 a | 91.75(15) | O3 a–Fe1–O12 a | 166.86(10) |
| O2–Fe1–O12 | 88.67(10) | O12–Fe1–O12 a | 79.66(15) |
| O2 a–Fe1–O12 | 96.89(10) |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benmansour, S.; Gómez-García, C.J. A Heterobimetallic Anionic 3,6-Connected 2D Coordination Polymer Based on Nitranilate as Ligand. Polymers 2016, 8, 89. https://doi.org/10.3390/polym8030089
Benmansour S, Gómez-García CJ. A Heterobimetallic Anionic 3,6-Connected 2D Coordination Polymer Based on Nitranilate as Ligand. Polymers. 2016; 8(3):89. https://doi.org/10.3390/polym8030089
Chicago/Turabian StyleBenmansour, Samia, and Carlos J. Gómez-García. 2016. "A Heterobimetallic Anionic 3,6-Connected 2D Coordination Polymer Based on Nitranilate as Ligand" Polymers 8, no. 3: 89. https://doi.org/10.3390/polym8030089
APA StyleBenmansour, S., & Gómez-García, C. J. (2016). A Heterobimetallic Anionic 3,6-Connected 2D Coordination Polymer Based on Nitranilate as Ligand. Polymers, 8(3), 89. https://doi.org/10.3390/polym8030089