Single-Crystal-to-Single-Crystal Anion Exchange in a Gadolinium MOF: Incorporation of POMs and [AuCl4]−
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of 1
2.2. Anion Exchange
Single crystal diffraction
2.3. Powder Diffraction
2.4. IR
2.5. EDAX
3. Results and Discussion
3.1. Crystal Structure Characterization
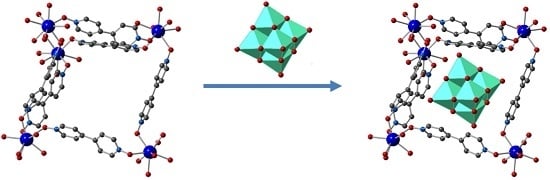
3.2. Anion Exchange
3.3. Location of Interchanged Anions
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MOF | Metal organic framework |
| POM | Polyoxomatalate |
| TfO | Triflate |
| MeOH | Methanol |
References
- Robson, R. A net-based approach to coordination polymers. J. Chem. Soc. Dalton Trans. 2000, 3735–3744. [Google Scholar] [CrossRef]
- Blake, A.J.; Champness, N.R.; Hubberstey, P.; Li, W.-S.; Withersby, M.A.; Schroder, M. Inorganic crystal engineering using self-assembly of tailored building-blocks. Coord. Chem. Rev. 1999, 183, 117–138. [Google Scholar] [CrossRef]
- Hoskins, B.F.; Robson, R. Infinite polymeric frameworks consisting of three dimensionally linked rod-like segments. J. Am. Chem. Soc. 1989, 111, 5962–5964. [Google Scholar] [CrossRef]
- Hoskins, B.F.; Robson, R. Design and construction of a new class of scaffolding-like materials comprising infinite polymeric frameworks of 3D-linked molecular rods. A reappraisal of the zinc cyanide and cadmium cyanide structures and the synthesis and structure of the diamond-rela. J. Am. Chem. Soc. 1990, 112, 1546–1554. [Google Scholar] [CrossRef]
- Zhou, H.-C.J.; Kitagawa, S. Metal Organic Frameworks. Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-C.J.; Long, J.R.; Yaghi, M.O. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Rowsell, J.L.C.; Yaghi, O.M. Metal-organic frameworks: A new class of porous materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.P.; Cheon, Y.E.; Lee, E.Y. Syntheses and functions of porous metallosupramolecular networks. Coord. Chem. Rev. 2008, 252, 1007–1026. [Google Scholar] [CrossRef]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar]
- James, S.L. Metal-organic frameworks. Chem. Soc. Rev. 2003, 32, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Janiak, C. Engineering coordination polymers towards applications. Dalton Trans. 2003, 14, 2781–2804. [Google Scholar] [CrossRef]
- Bradshaw, D.; Claridge, J.B.; Cussen, E.J.; Prior, T.J.; Rosseinsky, M.J. Design, chirality, and flexibility in nanoporous molecule-based materials. Acc. Chem. Res. 2005, 38, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M. Modifying MOFs: New chemistry, new materials. Chem. Sci. 2010, 1, 32–36. [Google Scholar] [CrossRef]
- Zhao, D.; Timmons, D.J.; Yuan, D.; Zhou, H.C. Tuning the topology and functionality of metal-organic frameworks by ligand design. Acc. Chem. Res. 2011, 44, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Dincă, M.; Long, J.R. Hydrogen storage in microporous metal-organic frameworks with exposed metal sites. Angew. Chem. Int. Ed. 2008, 47, 6766–6779. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, A.K.; Rao, C.N.R.; Feller, R.K. Structural diversity and chemical trends in hybrid inorganic organic framework materials. Chem. Commun. 2006. [Google Scholar] [CrossRef]
- Champness, N.R. The future of metal–organic frameworks. Dalton Trans. 2011, 40, 10311. [Google Scholar] [CrossRef] [PubMed]
- Brammer, L. Developments in inorganic crystal engineering. Chem. Soc. Rev. 2004, 33, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.E.; Platero-Prats, A.E.; Snejko, N.; Rojas, A.; Monge, A.; Gándara, F.; Gutiérrez-Puebla, E.; Camblor, M.A. Towards inorganic porous materials by design: Looking for new architectures. Adv. Mater. 2011, 23, 5283–5292. [Google Scholar] [CrossRef] [PubMed]
- Phan, A; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.J.; Dincă, M.; Long, J.R. Hydrogen storage in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1294–1314. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Jia, J.H.; Hubberstey, P.; Schroder, M.; Champness, N.R. Hydrogen storage in metal-organic frameworks. Crystengcomm 2007, 9, 438–448. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, W.; Qian, G.; Chen, B. Methane storage in metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 5657–5678. [Google Scholar] [CrossRef] [PubMed]
- Carrington, E.J.; Vitorica-Yrezabal, I.J.; Brammer, L. Crystallographic studies of gas sorption in metal-organic frameworks. Acta Crystallogr. Sect. B 2014, 70, 404–422. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal-organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Van de Voorde, B.; Bueken, B.; Denayer, J.; de Vos, D. Adsorptive separation on metal-organic frameworks in the liquid phase. Chem. Soc. Rev. 2014, 43, 5766–5788. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Cairns, A.J.; Liu, J.; Motkuri, R.K.; Nune, S.K.; Fernandez, C.A.; Krishna, R.; Strachan, D.M.; Thallapally, P.K. Potential of metal–organic frameworks for separation of xenon and krypton. Acc. Chem. Res. 2015, 48, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Abney, C.; Lin, W. Enantioselective catalysis with homochiral metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.-Y. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [PubMed]
- Zhang T., L.W. Metal–organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014, 5982–5993. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Garcia, H. Metal-organic frameworks as solid catalysts for the synthesis of nitrogen-containing heterocycles. Chem. Soc. Rev. 2014, 43, 5750–5765. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Marqués, M.; Hidalgo, T.; Serre, C.; Horcajada, P. Nanostructured metal–organic frameworks and their bio-related applications. Coord. Chem. Rev. 2016, 307, 342–360. [Google Scholar] [CrossRef]
- Coronado, E.; Mínguez Espallargas, G. Dynamic magnetic MOFs. Chem. Soc. Rev. 2013, 42, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M. Postsynthetic methods for the functionalization of metal–organic frameworks. Chem. Rev. 2012, 112, 970–1000. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cohen, S.M. Postsynthetic modification of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Ingleson, M.J.; Barrio, J.P.; Guilbaud, J.-B.; Khimyak, Y.Z.; Rosseinsky, M.J. Framework functionalisation triggers metal complex binding. Chem. Commun. 2008. [Google Scholar] [CrossRef] [PubMed]
- Burrows, A.D.; Frost, C.G.; Mahon, M.F.; Richardson, C. Post-synthetic modification of tagged metal-organic frameworks. Angew. Chem. Int. Ed. 2008, 47, 8482–8486. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.K.; Wang, Z.; Cohen, S.M. Systematic functionalization of a metal-organic framework via a postsynthetic modification approach. J. Am. Chem. Soc. 2008, 130, 8508–8517. [Google Scholar] [CrossRef] [PubMed]
- Nagai, A.; Guo, Z.; Feng, X.; Jin, S.; Chen, X.; Ding, X.; Jiang, D. Pore surface engineering in covalent organic frameworks. Nat. Commun. 2011, 2, 536. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Giménez-Marqués, M.; Mínguez Espallargas, G.; Brammer, L. Tuning the magneto-structural properties of non-porous coordination polymers by HCl chemisorption. Nat. Commun. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Juan-Alcañiz, J.; Gascon, J.; Kapteijn, F. Metal–organic frameworks as scaffolds for the encapsulation of active species: State of the art and future perspectives. J. Mater. Chem. 2012, 22, 10102–10118. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H. Hydrothermal synthesis of a metal-organic framework containing large rectangular channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H. T-shaped molecular building units in the porous structure of Ag(4,4′-bpy)·NO3. J. Am. Chem. Soc. 1996, 118, 295–296. [Google Scholar] [CrossRef]
- Noro, S.I.; Kitaura, R.; Kondo, M.; Kitagawa, S.; Ishii, T.; Matsuzaka, H.; Yamashita, M. Framework engineering by anions and porous functionalities of Cu(II)/4,4′-bpy coordination polymers. J. Am. Chem. Soc. 2002, 124, 2568–2583. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, L.; Ciani, G.; Maggini, S.; Proserpio, D.M.; Visconti, M. Heterometallic modular metal-organic 3D frameworks assembled via new tris-β-diketonate metalloligands: Nanoporous materials for anion exchange and scaffolding of selected anionic guests. Chem. A Eur. J. 2010, 16, 12328–12341. [Google Scholar] [CrossRef] [PubMed]
- Aijaz, A.; Lama, P.; Bharadwaj, P.K. Two-dimensional coordination polymer with a non-interpenetrated (4,4) net showing anion exchange and structural transformation in single-crystal-to-single-crystal fashion. Inorg. Chem. 2010, 49, 5883–5889. [Google Scholar] [CrossRef] [PubMed]
- Custelcean, R.; Moyer, B.A. Anion separation with metal-organic frameworks. Eur. J. Inorg. Chem. 2007, 1321–1340. [Google Scholar] [CrossRef]
- Fei, H.; Bresler, M.R.; Oliver, S.R.J. A new paradigm for anion trapping in high capacity and selectivity: Crystal-to-crystal transformation of cationic materials. J. Am. Chem. Soc. 2011, 133, 11110–11113. [Google Scholar] [CrossRef] [PubMed]
- Safarifard, V.; Morsali, A. Reversible crystal-to-crystal transformation of a 3D–3D coordination polymer by solid state anion-replacement with no change in nano-particle morphology. CrystEngComm 2011, 13, 4817–4819. [Google Scholar] [CrossRef]
- Fu, J.; Li, H.; Mu, Y.; Hou, H.; Fan, Y. Reversible single crystal to single crystal transformation with anion exchange-induced weak Cu2+···I− interactions and modification of the structures and properties of MOFs. Chem. Commun. 2011, 47, 5271–5273. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Guo, Y.M.; Chen, S.T.; Bu, X.H.; Batten, S.R.; Ribas, J.; Kitagawa, S. Preparation of acentric porous coordination frameworks from an interpenetrated diamondoid array through anion-exchange procedures: crystal structures and properties. Inorg. Chem. 2004, 43, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Liu, Z.; Li, Y.; Deng, H.; Zeng, R.; Zeller, M. Reversible anion exchange and sensing in large porous materials built from 4,4′-bipyridine via π⋯π and H-bonding interactions. Inorg. Chem. 2008, 47, 5122–5128. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Biradha, K. Interplay of hydrogen bonds in assembling (4,4)-coordination networks: Transformations from open to interpenetrated networks via anion exchange. Cryst. Growth Des. 2006, 6, 1742–1745. [Google Scholar] [CrossRef]
- Tzeng, B.-C.; Chiu, T.-H.; Chen, B.-S.; Lee, G.-H. Novel single-crystal-to-single-crystal anion exchange and self-assembly of luminescent d(10) metal (Cd(II), Zn(II), and Cu(I)) complexes containing C(3)-symmetrical ligands. Chemistry 2008, 14, 5237–5245. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Rosi, N.L. Tuning MOF CO2 adsorption properties via cation exchange. J. Am. Chem. Soc. 2010, 132, 5578–5579. [Google Scholar] [CrossRef] [PubMed]
- Calleja, G.; Botas, J.A.; Sánchez-Sánchez, M.; Orcajo, M.G. Hydrogen adsorption over Zeolite-like MOF materials modified by ion exchange. Int. J. Hydrog. Energy 2010, 35, 9916–9923. [Google Scholar] [CrossRef]
- Beer, P.D.; Gale, P.A. Anion recognition and sensing: The state of the art and future perspectives. Angew. Chem. Int. Ed. 2001, 40, 486–516. [Google Scholar] [CrossRef]
- Klet, R.C.; Tussupbayev, S.; Borycz, J.; Gallagher, J.R.; Stalzer, M.M.; Miller, J.T.; Gagliardi, L.; Hupp, J.T.; Marks, T.J.; Cramer, C.J.; et al. Single-site organozirconium catalyst embedded in a metal-organic framework. J. Am. Chem. Soc. 2015, 137, 15680–15683. [Google Scholar] [CrossRef] [PubMed]
- Grigoropoulos, A.; Whitehead, G.F.S.; Perret, N.; Katsoulidis, A.P.; Chadwick, F.M.; Davies, R.P.; Haynes, A.; Brammer, L.; Weller, A.S.; Xiao, J.; et al. Encapsulation of an organometallic cationic catalyst by direct exchange into an anionic MOF. Chem. Sci. 2016, 7, 2037–2050. [Google Scholar] [CrossRef]
- Kajiwara, T.; Fujii, M.; Tsujimoto, M.; Kobayashi, K.; Higuchi, M.; Tanaka, K.; Kitagawa, S. Photochemical reduction of low concentrations of CO2 in a porous coordination polymer with a Ruthenium(II)-CO Complex. Angew. Chem. Int. Ed. 2016, 55, 2697–2700. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Gimenez-Marques, M.; Gómez-García, C.J.; Mínguez Espallargas, G. Dynamic magnetic materials based on the cationic coordination polymer [Cu(btix)2]n2n+ [btix = 1,4-Bis(triazol-1-ylmethyl) benzene]: Tuning the structural and magnetic properties through anion exchange. Inorg. Chem. 2012, 51, 12938–12947. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Desai, A.V.; Ghosh, S.K. Ionic metal-organic frameworks (iMOFs): Design principles and applications. Coord. Chem. Rev. 2016, 307, 313–341. [Google Scholar] [CrossRef]
- Cronin, L.; Müller, A. From serendipity to design of polyoxometalates at the nanoscale, aesthetic beauty and applications. Chem. Soc. Rev. 2012, 41, 7333–7334. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.L. Introduction: Polyoxometalates-multicomponent molecular vehicles to porbe fundamental issues and practical problems. Chem. Rev. 1998, 98, 1–390. [Google Scholar] [CrossRef] [PubMed]
- Dolbecq, A.; Salomon, W.; Roch, C.; Mialane, P.; Rouschmeyer, P.; Serre, C.; Haouas, M.; Taulelle, F.; Yang, S.; Ruhlmann, L. Immobilization of polyoxometalates in the Zr-based metal organic framework UiO-67. Chem. Commun. 2015, 51, 2972–2975. [Google Scholar]
- Han, Q.; He, C.; Zhao, M.; Qi, B.; Niu, J.; Duan, C. Engineering chiral polyoxometalate hybrid metal-organic frameworks for asymmetric dihydroxylation of olefins. J. Am. Chem. Soc. 2013, 135, 10186–10189. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Naruke, H.; Yamase, T. A novel organic/inorganic hybrid nanoporous material incorporating Keggin-type polyoxometalates. Inorg. Chem. Commun. 2003, 6, 1020–1024. [Google Scholar] [CrossRef]
- Yan, A.-X.; Yao, S.; Li, Y.-G.; Zhang, Z.-M.; Lu, Y.; Chen, W.-L.; Wang, E.-B. Incorporating polyoxometalates into a porous MOF greatly improves its selective adsorption of cationic dyes. Chem. A Eur. J. 2014, 20, 6927–6933. [Google Scholar] [CrossRef] [PubMed]
- Bajpe, S.R.; Kirschhock, C.E.A.; Aerts, A.; Breynaert, E.; Absillis, G.; Parac-Vogt, T.N.; Giebeler, L.; Martens, J.A. Direct observation of molecular-level template action leading to self-assembly of a porous framework. Chem. A Eur. J. 2010, 16, 3926–3932. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-Y.; Liu, S.-X.; Liang, D.-D.; Shao, K.-Z.; Ren, Y.-H.; Su, Z.-M. Highly stable crystalline catalysts based on a microporous metal–organic framework and polyoxometalates. J. Am. Chem. Soc. 2009, 131, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Salomon, W.; Yazigi, F.-J.; Roch-Marchal, C.; Mialane, P.; Horcajada, P.; Serre, C.; Haouas, M.; Taulelle, F.; Dolbecq, A. Immobilization of Co-containing polyoxometalates in MIL-101(Cr): Structural integrity versus chemical transformation. Dalton Trans. 2014, 43, 12698–12705. [Google Scholar] [CrossRef] [PubMed]
- Mellot-Draznieks, C.; Dutour, J.; Férey, G. Hybrid organic-inorganic frameworks: Routes for computational design and structure prediction. Angew. Chem. Int. Ed. 2004, 43, 6290–6296. [Google Scholar] [CrossRef] [PubMed]
- Maksimchuk, N.V.; Timofeeva, M.N.; Melgunov, M.S.; Shmakov, A.N.; Chesalov, Y.A.; Dybtsev, D.N.; Fedin, V.P.; Kholdeeva, O.A. Heterogeneous selective oxidation catalysts based on coordination polymer MIL-101 and transition metal-substituted polyoxometalates. J. Catal. 2008, 257, 315–323. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; Barbosa, A.D.S.; Silva, P.; Paz, F.A.A.; Saini, V.K.; Pires, J.; de Castro, B.; Balula, S.S.; Cunha-Silva, L. Monovacant polyoxometalates incorporated into MIL-101(Cr): Novel heterogeneous catalysts for liquid phase oxidation. Appl. Catal. A 2013, 453, 316–326. [Google Scholar] [CrossRef]
- Baldoví, J.J.; Coronado, E.; Gaita-Ariño, A.; Gamer, C.; Giménez-Marqués, M.; Mínguez Espallargas, G. A SIM-MOF: Three-dimensional organisation of single-ion magnets with anion-exchange capabilities. Chem. A Eur. J. 2014, 20, 10695–10702. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, A.P. (Ed.) Inorganic Syntheses; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1990; Volume 27.
- Flynn, C.M.; Pope, M.T. Tungstovanadate heteropoly complexes. I. Vanadium(V) complexes with the constitution M6O19n- and vanadium:tungsten. leq. 1:2. Inorg. Chem. 1971, 10, 2524–2529. [Google Scholar] [CrossRef]
- Hori, T.; Himeno, S.; Tamada, O. Crystal structure of bis(tetra-n-butylammonium)dodecamolybdosulfate(VI)-(2-), [NBun4]2[SMo12O40]. J. Chem. Soc. Dalton Trans. 1996. [Google Scholar] [CrossRef]
- Meilikhov, M.; Yusenko, K.; Esken, D.; Turner, S.; van Tendeloo, G.; Fischer, R.A. Metals@MOFs—Loading MOFs with metal nanoparticles for hybrid functions. Eur. J. Inorg. Chem. 2010, 2010, 3701–3714. [Google Scholar] [CrossRef]
- Zordan, F.; Mínguez Espallargas, G.; Brammer, L. Unexpected structural homologies involving hydrogen-bonded and halogen-bonded networks in halopyridinium halometallate salts. CrystEngComm 2006, 8, 425–431. [Google Scholar] [CrossRef]







| Compound | 1 | 1–W6O19 | 1–Mo6O19 |
|---|---|---|---|
| Empirical formula | C129H96F27Gd3N24O51S9 | C123H96F9Gd3N24O90S3W18 | C123H96F9Gd3Mo18N24O90S3 |
| Formula weight | 4,071.59 | 7,398.47 | 5,816.09 |
| Crystal color | Colorless | Colorless | Yellow |
| Crystal size (mm3) | 0.13 × 0.11 × 0.06 | 0.10 × 0.08 × 0.06 | 0.17 × 0.11 × 0.05 |
| Temperature (K) | 120(2) | 120(2) | 120(2) |
| Crystal system, Z | Triclinic, 2 | Triclinic, 2 | Triclinic, 2 |
| Space group | P | P | P |
| a (Å) | 24.0370(8) | 24.0555(13) | 24.0547(9) |
| b (Å) | 24.0747(8) | 24.0908(6) | 24.1561(9) |
| c (Å) | 24.4414(7) | 24.6766(8) | 24.6456(6) |
| α (°) | 85.291(2) | 86.126(2) | 86.253(2) |
| β (°) | 62.188(3) | 61.429(4) | 61.542(3) |
| γ (°) | 61.627(3) | 61.276(4) | 61.515(4) |
| V (Å3) | 10,828.6(6) | 10,766.3(7) | 10,820.5(6) |
| ρcalc (mg/m3) | 1.249 | 2.282 | 1.785 |
| μ(MoKα) (mm−1) | 1.085 | 10.600 | 2.030 |
| θ range (°) | 3.24–25.05 | 2.91–25.04 | 2.85–25.06 |
| Reflns collected | 80,917 | 83,116 | 186,594 |
| Independent reflns (Rint) | 38,196(0.0717) | 37,930(0.1141) | 38,229(0.1116) |
| Reflns used in refinement, n | 38,196 | 37,930 | 38,229 |
| L. S. parameters, p/restraints, r | 1,659/74 | 864/198 | 1,841/128 |
| R1(F),a I > 2σ(I) | 0.1347 | 0.1509 | 0.1687 |
| wR2(F2),b all data | 0.4321 | 0.4559 | 0.4969 |
| S(F2),c all data | 1.503 | 1.244 | 1.866 |
| Compound | 1–AuCl4 | 1–W6O19–AuCl4 |
|---|---|---|
| Empirical formula | C120H96Au7.50Cl31.50Gd3N24O25 | C120H96Au1.50Cl9Gd3N24O66.25W13.5 |
| Formula weight | 5,339.88 | 6,510.43 |
| Crystal color | Yellow | Yellow |
| Crystal size (mm3) | 0.07 × 0.07 × 0.05 | 0.08 × 0.06 × 0.06 |
| Temperature (K) | 120(2) | 120(2) |
| Crystal system, Z | Triclinic, 2 | Triclinic, 2 |
| Space group | P | P |
| a (Å) | 23.9544(4) | 23.8865(16) |
| b (Å) | 24.3447(4) | 24.1640(15) |
| c (Å) | 24.8313(4) | 24.5613(16) |
| α (°) | 71.2790(10) | 86.866(5) |
| β (°) | 62.647(2) | 61.457(7) |
| γ (°) | 60.725(2) | 61.595(6) |
| V (Å3) | 11,132.0(3) | 10,677.4(12) |
| ρcalc (mg/m3) | 1.593 | 2.025 |
| μ(MoKα) (mm−1) | 6.229 | 9.364 |
| θ range (°) | 3.23–25.08 | 3.27–25.05 |
| Reflns collected | 241,553 | 131,931 |
| Independent reflns (Rint) | 39,355(0.1234) | 37,696(0.2606) |
| Reflns used in refinement, n | 39,355 | 37,696 |
| L. S. parameters, p/restraints, r | 910/24 | 681/98 |
| R1(F),a I>2σ(I) | 0.1489 | 0.2284 |
| wR2(F2),b all data | 0.4541 | 0.5898 |
| S(F2),c all data | 1.768 | 1.482 |
| 1 (T) | 1 (E) | 1–W6O19 (T) | 1–W6O19 (E) | 1–AuCl4 (T) | 1–AuCl4 (E) | 1–W6O19-AuCl4 (T) | 1–W6O19–AuCl4 (E) | |
|---|---|---|---|---|---|---|---|---|
| Gd | 1 | 1 | 1 | 1.2 | 1 | 1 | 1 | 2.4 |
| S | 3 | 3.4 | 1 | 1.0 | – | – | – | – |
| W | – | – | 6 | 6.02 | – | – | 6 | 10 |
| Au | – | – | – | – | 3 | 3.12 | 1 | 1 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Cabrelles, J.; Mínguez Espallargas, G.; Coronado, E. Single-Crystal-to-Single-Crystal Anion Exchange in a Gadolinium MOF: Incorporation of POMs and [AuCl4]−. Polymers 2016, 8, 171. https://doi.org/10.3390/polym8050171
López-Cabrelles J, Mínguez Espallargas G, Coronado E. Single-Crystal-to-Single-Crystal Anion Exchange in a Gadolinium MOF: Incorporation of POMs and [AuCl4]−. Polymers. 2016; 8(5):171. https://doi.org/10.3390/polym8050171
Chicago/Turabian StyleLópez-Cabrelles, Javier, Guillermo Mínguez Espallargas, and Eugenio Coronado. 2016. "Single-Crystal-to-Single-Crystal Anion Exchange in a Gadolinium MOF: Incorporation of POMs and [AuCl4]−" Polymers 8, no. 5: 171. https://doi.org/10.3390/polym8050171
APA StyleLópez-Cabrelles, J., Mínguez Espallargas, G., & Coronado, E. (2016). Single-Crystal-to-Single-Crystal Anion Exchange in a Gadolinium MOF: Incorporation of POMs and [AuCl4]−. Polymers, 8(5), 171. https://doi.org/10.3390/polym8050171