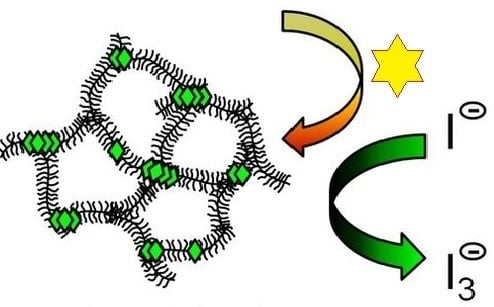
Porphyrin Diacid-Polyelectrolyte Assemblies: Effective Photocatalysts in Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. UV/Vis Spectroscopy
2.4. Atomic Force Microscopy
2.5. Small Angle Neutron Scattering (SANS)
3. Results
3.1. Atomic Force Microscopy (AFM)
3.2. Structural Investigation by Small Angle Neutron Scattering (SANS)
3.3. Spectroscopic Investigation
3.4. Catalysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, J.; Xiao, F.; Xiao, G.; Liu, B. Assembly of a CdS quantum dot-TiO2 nanobelt heterostructure for photocatalytic application: Towards an efficient visible light photocatalyst via facile surface charge tuning. New J. Chem. 2015, 39, 279–286. [Google Scholar] [CrossRef]
- Lin, Z.-Q.; Sun, P.-J.; Tay, Y.-Y.; Liang, J.; Liu, Y.; Shi, N.-E.; Xie, L.-H.; Yi, M.-D.; Qian, Y.; Fan, Q.-L.; et al. Kinetically controlled assembly of a spirocyclic aromatic hydrocarbon into polyhedral micro/nanocrystals. ACS Nano 2012, 6, 5309–5319. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xiao, J.; Wong, J.I.; Guo, J.; Wu, Y.; Ong, L.; Lao, L.L.; Boey, F.; Zhang, H.; Yang, H.Y.; et al. Shape-controlled micro/nanostructures of 9,10-diphenylanthracene (DPA) and their application in light-emitting devices. J. Phys. Chem. C 2011, 115, 7924–7927. [Google Scholar] [CrossRef]
- Takacs, C.T.; Sun, Y.; Welch, G.C.; Perez, L.A.; Liu, X.; Wen, W.; Bazan, G.C.; Heeger, A.J. Solar cell efficiency, self-assembly, and dipole-dipole interactions of isomorphic narrow-band-gap molecules. J. Am. Chem. Soc. 2012, 134, 16597–16606. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Yin, Z.; Wu, Y.; Guo, J.; Cheng, Y.; Li, H.; Huang, Y.; Zhang, Q.; Ma, J.; Boey, F.; et al. Chemical reaction between Ag Nanoparticles and TCNQ microparticles in aqueous solution. Small 2011, 7, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Busseron, E.; Ruff, Y.; Moulin, E.; Giuseppone, N. Supramolecular self-assemblies as functional nanomaterials. Nanoscale 2013, 5, 7098–7140. [Google Scholar] [CrossRef] [PubMed]
- Förster, S.; Plantenberg, T. Funktionale Strukturhierarchien aus selbstorganisierenden polymeren. Angew. Chem. 2002, 114, 712–739. [Google Scholar] [CrossRef]
- Philp, D.; Stoddart, J.F. Self-assembly in natural and unnatural systems. Angew. Chem. 1996, 108, 1242–1286. [Google Scholar] [CrossRef]
- Schneider, H.J. Bindungsmechanismen in supramolekularen Komplexen. Angew. Chem. 2009, 121, 3982–4036. [Google Scholar] [CrossRef]
- Ikkala, O.; Brinke, B. Functional materials based on self-assembly of polymeric supramolecules. Science 2002, 295, 2407–2409. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Grzybowski, B. Self-assembly at all scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef] [PubMed]
- Marsden, H. R.; Kros, A. Self-assembly of coiled coils in synthetic biology: Inspiration and progress. Angew. Chem. Int. Ed. 2010, 49, 2988–3005. [Google Scholar] [CrossRef] [PubMed]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar]
- Donath, E.; Sukhorukov, G.B.; Caruso, F.; Davis, S.A.; Möhwald, H. Novel hollow polymer shells by colloid-templated assembly of polyelectrolytes. Angew. Chem. Int. Ed. 1998, 37, 2202. [Google Scholar] [CrossRef]
- Bertrand, P.; Jones, A.; Laschewsky, A.; Legras, R. Ultrathin polymer coatings by complexation of polyelectrolytes at interfaces: Suitable materials, structure and properties. Macromol. Rapid Commun. 2000, 21, 319. [Google Scholar] [CrossRef]
- Glinel, K.; Dejugnat, C.; Prevot, M.; Schöler, B.; Schönhoff, M.; von Klitzing, R. Responsive polyelectrolyte multilayers. Colloids Surf. A 2007, 303, 3. [Google Scholar] [CrossRef]
- Antonietti, M.; Conrad, J.; Thünemann, A. Polyelectrolyte-surfactant complexes: A New type of solid, mesomorphous material. Macromolecules 1994, 27, 6007. [Google Scholar] [CrossRef]
- Rädler, J.O.; Koltover, I.; Salditt, T.; Safinya, C.R. Structure of DNA-cationic liposome complexes: DNA intercalation in multilamellar membranes in distinct interhelical packing regimes. Science 1997, 275, 810. [Google Scholar] [CrossRef] [PubMed]
- Faul, C.F. J.; Antonietti, M. Ionic self-assembly: Facile synthesis of supramolecular materials. Adv. Mater. 2003, 15, 673. [Google Scholar] [CrossRef]
- Zakrevskyy, Y.; Stumpe, J.; Smarsly, B.; Faul, C.F. Photoinduction of optical anisotropy in an azobenzene-contining ionic self-assembly liquid-crystalline material. J. Phys. Rev. E 2007, 75, 031703. [Google Scholar] [CrossRef] [PubMed]
- Thünemann, F.; Müller, M.; Dautzenberg, H.; Joanny, H.F.O.; Löwen, H. Polyelectrolyte complexes. Adv. Polym. Sci. 2004, 166, 113. [Google Scholar]
- Cooper, C.L.; Dubin, P.L.; Kayitmazer, A.B.; Turksen, S. Polyelectrolyte-protein complexes. Curr. Opin. Colloid Interface. Sci. 2005, 10, 52. [Google Scholar] [CrossRef]
- Müller, M.; Kessler, B.; Richter, S. Preparation of monomodal polyelectrolyte complex nanoparticles of PDADMAC/poly(maleic acid-alt-α-methylstyrene) by consecutive centrifugation. Langmuir 2005, 21, 7044. [Google Scholar] [CrossRef] [PubMed]
- Böhme, U.; Scheler, U. Hydrodynamic size and charge of polyelectrolyte complexes. J. Phys. Chem. B 2007, 111, 8348. [Google Scholar] [CrossRef] [PubMed]
- Gröhn, F.; Klein, K.; Brand, S. Facile route to supramolecular structures: Self-assembly of dendrimers and naphthalene dicarboxylic acids. Chem. Eur. J. 2008, 14, 6866–6889. [Google Scholar] [CrossRef] [PubMed]
- Ruthardt, C.; Maskos, M.; Kolb, U.; Gröhn, F. Finite-size networks from cylindrical polyelectrolyte brushes and porphyrins. Macromolecules 2009, 42, 830–840. [Google Scholar] [CrossRef]
- Willerich, I.; Gröhn, F. Photoswitchable nanoassemblies by electrostatic self-assembly. Angew. Chem. Int. Ed. 2010, 44, 8104. [Google Scholar] [CrossRef] [PubMed]
- Gröhn, F.; Klein, K.; Koynov, K. A novel type of vesicles based on ionic and π-π interactions. Macromol. Rapid Commun. 2010, 31, 75. [Google Scholar] [CrossRef] [PubMed]
- Willerich, I.; Gröhn, F. Molecular structure encodes nanoscale assemblies: Understanding driving forces in electrostatic self-assembly. J. Am. Chem. Soc. 2011, 133, 20341–20356. [Google Scholar] [CrossRef] [PubMed]
- Ruthardt, C.; Maskos, M.; Kolb, U.; Gröhn, F. Polystyrene sulfonate-porphyrin assemblies: Influence of polyelectrolyte and porphyrin structure. J. Phys. Chem. B 2011, 115, 5716–5729. [Google Scholar] [CrossRef] [PubMed]
- Frühbeißer, S.; Gröhn, F. Catalytic activity of macroion-porphyrin nanoassemblies. J. Am. Chem. Soc. 2012, 134, 14267–14270. [Google Scholar] [CrossRef] [PubMed]
- Düring, J.; Hölzer, A.; Kolb, U.; Branscheid, R.; Gröhn, F. Supramolecular organic-inorganic hybrid assemblies with tunable particle size: Interplay of three noncovalent interactions. Angew. Chem. Int. Ed. 2013, 52, 8742–8745. [Google Scholar] [CrossRef] [PubMed]
- Moldenhauer, D.; Gröhn, F. Nanoassemblies with light-responsive size and density from linear flexible polyelectrolytes. J. Polym. Sci. B 2013, 51, 802–816. [Google Scholar] [CrossRef]
- Hasobe, T.; Fukuzumi, S. Nanostructured assembly of porphyrin clusters for light energy conversion. J. Mater. Chem. 2003, 13, 2515–2520. [Google Scholar] [CrossRef]
- Merchat, M.; Bertolini, G.; Giacomini, P. Meso-substituted cationic porphyrins as efficient photosensitizers of Gram-positive and Gram-negative bacteria. J. Photochem. Photobiol. B 1996, 32, 153–157. [Google Scholar] [CrossRef]
- Milanesio, M.E.; Alvarez, M.G.; Bertolotti, S.G. Photophysical characterization and photodynamic activity of metallo 5-(4- (trimethylammonium)phenyl)-10,15,20-tris(2,4,6-trimethoxyphenyl)porphyrin in homogeneous and biomimetic media. Photochem. Photobiol. Sci. 2008, 7, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, E.D.; Alvarez, M.G.; Durantini, E.N. Susceptibility of Candida albicans to photodynamic action of 5,10,15,20-tetra(4-N-methylpyridyl)porphyrin in different media. FEMS Immunol. Med. Microbiol. 2010, 60, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Ethirajan, M.; Chen, Y.; Joshi, P. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef] [PubMed]
- Afonso, S.G.; Salamanca, R.; Batlle, A.M. The photodynamic and non-photodynamic actions of porphyrins. Braz. J. Med. Biol. Res. 1999, 32, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W. Photodynamic therapy. J. Nat.Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Kalyanasundaram, K.; Neumann-Spalart, M. Photophysical and redox properties of water-soluble porphyrins in aqueous media. J. Phys. Chem. 1982, 86, 5163–5169. [Google Scholar] [CrossRef]
- Kubat, P.; Mosinger, J. Photophysical properties of metal complexes of meso-tetrakis (4-sulphonatophenyl) porphyrin. J. Photochem. Photobiol. A 1996, 96, 93–97. [Google Scholar] [CrossRef]
- Kee, H.L.; Bhaumik, J.; Diers, J.R.; Mroz, P.; Hamblin, M.R. Photophysical characterization of imidazolium-substituted Pd(II), In(III), and Zn(II) porphyrins as photosensitizers for PDT. J. Photochem. Photobiol. A 2008, 200, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.W.; Smith, R.; Robinson, R.; Robins, M. Photophysical properties of porphyrins, phthalocyanines, and benzochlorins. Inorg. Chim. Acta 1998, 279, 226–231. [Google Scholar] [CrossRef]
- Prochazkova, K.; Zelinger, Z.; Lang, K.; Kubat, P. meso-Tetratolylporphyrins substituted by pyridinium groups: Aggregation, photophysical properties and complexation with DNA. J. Phys. Org. Chem. 2004, 17, 890–897. [Google Scholar] [CrossRef]
- Ricchelli, F. Photophysical properties of porphyrins in biological membranes. J. Photochem. Photobiol. B 1995, 29, 109–118. [Google Scholar] [CrossRef]
- Sun, W.-J.; Li, J.; Mele, G.; Zhang, Z.-Q.; Zhang, F.-X. Enhanced photocatalytic degradation of rhodamine B by surface modification of ZnO with copper (II) porphyrin under both UV–Vis and visible light irradiation. J. Mol. Catal. A 2013, 233, 84–91. [Google Scholar] [CrossRef]
- Ishihara, S.; Labuta, J.; Van Rossom, W.; Ishikawa, D.; Minami, K.; Hill, J.P.; Ariga, K. Porphyrin-based sensor nanoarchitectonics in diverse physical detection modes. Phys. Chem. Chem. Phys. 2014, 16, 9713. [Google Scholar] [CrossRef] [PubMed]
- Nakazono, T.; Parent, A.R.; Sakai, K. Cobalt porphyrins as homogeneous catalysts for water oxidation. Chem. Commun. 2013, 49, 6325–6327. [Google Scholar] [CrossRef] [PubMed]
- Windle, C.D.; Campian, M.V.; Duhme-Klair, A.-K.; Gibson, E.A.; Perutz, R.N.; Schneider, J. CO2 photoreduction with long-wavelength light: Dyads and monomers of zinc porphyrin and rhenium bipyridine. Chem. Commun. 2012, 48, 8189–8191. [Google Scholar] [CrossRef] [PubMed]
- Urbani, M.; Grätzel, M.; Nazeeruddin, M.K.; Torres, T. Meso-substituted porphyrins for dye-sensitized solar cells. Chem. Rev. 2014, 114, 12330–12396. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, L.; Liu, F.; Li, W.; Chen, R.; Gao, Y.; Zhang, W. Photodynamic therapy of oligoethylene glycol dendronized reduction-sensitive porphyrins. J. Mater. Chem. B 2015, 3, 3062–3071. [Google Scholar] [CrossRef]
- Labuta, J.; Hill, J.P.; Ishihara, S.; Hanykova, L.; Ariga, K. Chiral sensing by nonchiral tetrapyrroles. Acc. Chem. Res. 2015, 48, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, L.; Bai, H.; Hong, W.; Li, C.; Shi, G. Chemically converted graphene induced molecular flattening of 5,10,15,20-tetrakis(1-methyl-4-pyridino)porphyrin and its application for optical detection of cadmium(II) ions. J. Am. Chem. Soc. 2009, 131, 13490–13497. [Google Scholar] [CrossRef] [PubMed]
- Kano, K.; Minamizono, H.; Kitae, T.; Negi, S. Self-aggregation of cationic porphyrins in water. Can π–π stacking interaction overcome electrostatic repulsive force? J. Phys. Chem. A 1997, 101, 6118–6124. [Google Scholar] [CrossRef]
- Iosif, A. Aggregation of tetrakis(4-methylpyridy1)porphyrin and tetrakis(4-sulphonatopheny1)porphyrin in water. J. Prakt. Chem. 1997, 339, 420–425. [Google Scholar] [CrossRef]
- Pasternack, R.F. Aggregation properties of water-soluble porphyrins. Ann. N. Y. Acad. Sci. 1973, 206, 614–630. [Google Scholar] [CrossRef] [PubMed]
- Lauceri, R.; de Napoli, M.; Mammana, A.; Nardis, S. Hierarchical self-assembly of water-soluble porphyrins. Synth. Met. 2004, 147, 49–55. [Google Scholar] [CrossRef]
- Kano, K.; Takei, M.; Hashimoto, S. Cationic porphyrins in water. 1H NMR and fluorescence studies on dimer and molecular complex formation. J. Phys. Chem. 1990, 94, 2181–2187. [Google Scholar] [CrossRef]
- Pasternack, R.F.; Huber, P.R.; Boyd, P. On the aggregation of meso-substituted water-soluble porphyrins. J. Am. Chem. Soc. 1972, 94, 4511–4517. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Romeo, A.; Villari, V.; Micali, N.; Foltran, I.; Foresti, E.; Lesci, I.G.; Roveri, N.; Zuccheri, T.; Monsu` Scolaro, L. Self-organizing functional materials via ionic self assembly: Porphyrins hand J-aggregates on synthetic chrysotile nanotubes. J. Am. Chem. Soc. 2009, 131, 6920–6921. [Google Scholar] [CrossRef] [PubMed]
- Elemans, J.A. A.W.; van Hameren, R.; Nolte, R.J. M.; Rowan, A.E. Molecular materials by self-assembly of porphyrins, phthalocyanines, and perylenes. Adv. Mater. 2006, 18, 1251–1266. [Google Scholar] [CrossRef]
- Tu, S.; Kim, S.H.; Joseph, J.; Modarelli, D.A.; Parquette, J.R. Self-assembly of a donor-acceptor nanotube. A strategy to create bicontinuous arrays. J. Am. Chem. Soc. 2011, 133, 19125–19130. [Google Scholar] [CrossRef] [PubMed]
- Vilaplana, R.A.; Gonzalez-Vilcheza, F.; Pasternack, R.F. Formation of supramolecules in solution. interaction between transition-metal complexes and water-soluble porphyrins. J. Chem. Soc. Dalton Trans. 1991, 1831–1834. [Google Scholar] [CrossRef]
- Taggart, J.C.; Welch, E.Z.; Mulqueen, M.F.; Dioguardi, V.B.; Cauer, A.G.; Kokona, B.; Fairman, R. Testing the role of charge and structure on the stability of peptide−porphyrin complexes. Biomacromolecules 2014, 15, 4544–4550. [Google Scholar] [CrossRef] [PubMed]
- Shema-Mizrachi, M.; Pavan, G.M.; Levin, E.; Danani, A.; Lemcoff, N.G. Catalytic chameleon dendrimers. J. Am. Chem. Soc. 2011, 133, 14359–14367. [Google Scholar] [CrossRef] [PubMed]
- Asha Jhonsi, M.; Renganathan, R. Investigations on the photoinduced interaction of water-soluble thioglycolic acid (TGA) capped CdTe quantum dots with certain porphyrins. J. Colloid Interface Sci. 2010, 344, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Amaoa, Y.; Tomonoua, Y.; Okura, I. Highly efficient photochemical hydrogen production system using zinc porphyrin and hydrogenase in CTAB micellar system. Sol. Energy Mater. Sol. Cells 2003, 79, 103–111. [Google Scholar] [CrossRef]
- Kellett, R.M.; Spiro, T.G. Cobalt (I) porphyrin catalysis of hydrogen production from water. Inorg. Chem. 1985, 24, 2373–2377. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Grätzel, M. Light induced redox reactions of water soluble porphyrins, sensitization of hydrogen generation from water by zincporphyrin derivatives. Helv. Chim. Acta 1980, 63, 478–485. [Google Scholar] [CrossRef]
- Tian, Y.; Martin, K.E.; Shelnutt, J.Y.-T.; Evans, L.; Busani, T.; Miller, J.E.; Medforth, C.J.; Shelnutt, J.A. Morphological families of self-assembled porphyrin structures and their photosensitization of hydrogen generation. Chem. Commun. 2011, 47, 6069–6071. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, M.; Zhang, S.; Pan, J.; Na, Y.; Liu, J.; Akermark, B.; Sun, L. Noncovalent assembly of metalloporphyrin and an iron hydrogenase active-site model: Photo-induced electron transfer and hydrogen generation. J. Phys. Chem. B 2008, 112, 8198–8202. [Google Scholar] [CrossRef] [PubMed]
- Udal’tsov, A.V. Initial steps of photosynthetic water splitting by associates of porphyrin. J. Photochem. Photobiol. A 2000, 130, 21–33. [Google Scholar] [CrossRef]
- Esswein, A.J.; Nocera, D.G. Hydrogen production by molecular photocatalysis. Chem. Rev. 2007, 107, 4022–4047. [Google Scholar] [CrossRef] [PubMed]
- Darwent, J.R.; Douglas, P.; Harriman, A.; Porter, G.; Richoux, M.-C. Metal phthalocyanines as photosensitizers for reduction of water to hydrogen. Coord. Chem. Rev. 1982, 44, 83–126. [Google Scholar] [CrossRef]
- Harriman, A.; Richoux, M.-C. Photoproduction of hydrogen from reductive quenching of a water-soluble zinc porphyrin. J. Photochem. 1981, 15, 336–339. [Google Scholar] [CrossRef]
- Sandanayaka, A.S.D.; Murakami, T.; Hasobe, T. Preparation and photophysical and photoelectrochemical properties of supramolecular porphyrin nanorods structurally controlled by encapsulated-fullerene derivatives. J. Phys. Chem. C 2009, 113, 18369–18378. [Google Scholar] [CrossRef]
- Harriman, A.; Richoux, M.-C. Luminescence of poprhyrins and metalloporphyrins VIII: Luminescence and hydrogen photogeneration from porphyrin conjugate diacids. J. Photochem. 1984, 27, 205–214. [Google Scholar] [CrossRef]
- Fleischer, E.B. The structure of porphyrins and metalloporhyrins. Acc. Chem. Res. 1970, 3, 105–112. [Google Scholar] [CrossRef]
- Kruk, M.M.; Starukhin, A.S.; Maes, W. Influence of macrocycle protonation on the photophysical properties of porphyrins. Macroheterocycles 2011, 4, 69–79. [Google Scholar] [CrossRef]
- Stone, A.; Fleischer, E.B. The molecular and crystal structure of porphyrin diacids. J. Am. Chem. Soc. 1968, 90, 2735–2748. [Google Scholar] [CrossRef]
- Dziezok, P.; Sheiko, S.S.; Fischer, K.; Schmidt, M.; Möller, M. Cylindrical molecular brushes. Angew. Chem. Int. Ed. Engl. 1997, 36, 2812–2815. [Google Scholar] [CrossRef]
- Note: This green colour leads to problems for the common structural investigation with dynamic light scattering due to absorption of the light of the whole visible spectrum. Consequently dynamic light scattering cannot be used.
- Kalyanasundaram, K. Photochemistry of water-soluble porphyrins: Comparative study of isomeric tetrapyridyl- and tetrakis(N-methylpyridiniumyl)porphyrins. Inorg. Chem. 1984, 23, 2453–2459. [Google Scholar] [CrossRef]
- Kalyanasundaram, K. Photochemistry and sensitized evolution of hydrogen from water using water-soluble cationic porphyrins. J. Chem. Soc. Faraday Trans. 2 1983, 79, 1365–1374. [Google Scholar] [CrossRef]
- Wang, L.-F.; Meng, X.-W.; Tang, F.-Q. Density functional theory study of electronic absorption spectra and intermolecular interactions of porphyrin–borate complexes. J. Mol. Struct.: THEOCHEM 2010, 956, 26–32. [Google Scholar] [CrossRef]
- Cho, S.; Lee, J.; Han, H.S.; Lee, H.K.; Jeon, S. Characteristic electronic perturbation by asymmetric arrangements of p-aminophenyl substituents in free-base porphyrins. J. Phys. Chem. A 2014, 118, 4995–5001. [Google Scholar] [CrossRef] [PubMed]












| Sample | RGC 1 (nm) | Diameter 1 (nm) | Model | Length (nm) | Radius (nm) |
|---|---|---|---|---|---|
| PSSbrush neutral | 4.8 | 13.8 | Cylinder | 140 ± 10 | 5.9 ± 0.1 |
| TMpyP + PSSbrush | 4 | 11.4 | Cylinder | 100 ± 10 | 5.1 ± 0.1 |
| TMPyPmonoacid + PSSbrush l = 0.4 | 4.0 | 11.4 | Cylinder | 93 ± 3 | 4.8 ± 0.1 |
| TMPyP diacid + PSS brush l = 0.4 | – | – | Cylinder | 2,700 * | 12 ± 0.1 |
| Zn-TMPyP + PSS brush l = 0.4 | 4.6 | 13 | Cylinder | 200 * | 5.8 ± 0.1 |
| TAPP diacid + PSSbrush l = 0.4 | 4.3 | 12.3 | Cylinder | 100 * | 5.2 ± 0.1 |
| System | Soret-Band (nm) | Qy(1,0) (nm) | Qy(0,0) (nm) | Qx(1,0) (nm) | Qx(0,0) (nm) |
|---|---|---|---|---|---|
| TAPP diacid | 432 | 589 | 640 | – | – |
| TAPP diacid l = 0.4 | 438 | 594 | 647 | – | – |
| TAPP | 412 | 514 | 549 | 579 | 634 |
| TAPP l = 0.4 | 421 | 516 | 549 | 588 | 644 |
| TMPyP diacid | 445 | 591 | 642 | – | – |
| TMPyp diacid l = 0.1 | 450 | 595 | 647 | – | – |
| TMPyp monoacid | 423 | 518 | 558 | 586 | |
| TMPyP monoacid l = 0.4 | 430/447 | 518 | 554 | 592 | 644 |
| TMPyp | 422 | 519 | 555 | 584 | 640 |
| TMPyP l = 0.4 | 424 | 520 | 555 | 591 | 646 |
| Zn-TMPyP acidic | 428 | 519 | 563 | – | – |
| Zn-TMPyP acidic l = 0.4 | 445 | 518 | 566/591/645 | – | – |
| Zn-TMPyP | 436 | 562 | 606 | – | – |
| Zn-TMPyP l = 0.4 | 442 | 566 | 608 | – | – |
| Porphyrin species | With PE | Without PE | Activity increase with PE |
|---|---|---|---|
| TMPyP diacid | 4.2 × 104 | 4.2 × 104 | unchanged |
| TAPP diacid | 1.5 × 104 | 5.9 × 105 | 2.5× |
| Zn-TMPyP | 8.7 × 105 | 6.1 × 105 | 1.3× |
| TMPyP monoacid | 7.8 × 105 | 2.3 × 105 | 3.4× |
| Porphyrin species | TON | TOF/min−1 | ||
|---|---|---|---|---|
| With PE | Without PE | With PE | Without PE | |
| TMPyP diacid | 109 | 104 | 1.82 | 1.73 |
| TAPP diacid | 20 | 6 | 0.66 | 0.1 |
| TAPPneutral | 2 | – | 0.03 | – |
| Zn-TMPyP | 8 | 47 | 0.14 | 0.8 |
| TMPyP monoacid | 10 | 3 | 0.14 | 0.04 |
| TMPyP neutral | 4 | 2 | 0.06 | 0.04 |
| Porphyrin species | With PE | Without PE | Increased activity with PE |
|---|---|---|---|
| TMPyP diacid | 9.42 × 104 | 1.00 × 103 | slightly less catalytically active |
| TAPP diacid | 5.26 × 104 | 7.01 × 105 | 7.5× |
| Zn-TMPyP | 2.59 × 104 | 7.73 × 105 | 3.6× |
| TMPyP monoacid | 2.01 × 104 | 7.35 × 105 | 2.7× |
| Porphyrin species | TON | TOF/min−1 | ||
|---|---|---|---|---|
| With PE | Without PE | With PE | Without PE | |
| TMPyP diacid | 243 | 246 | 0.81 | 0.82 |
| TAPP diacid | 78 | 10 | 0.25 | 0.03 |
| Zn-TMPyP | 33 | 9 | 0.11 | 0.03 |
| TMPyP monoacid | 46 | 16 | 0.15 | 0.05 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frühbeißer, S.; Mariani, G.; Gröhn, F. Porphyrin Diacid-Polyelectrolyte Assemblies: Effective Photocatalysts in Solution. Polymers 2016, 8, 180. https://doi.org/10.3390/polym8050180
Frühbeißer S, Mariani G, Gröhn F. Porphyrin Diacid-Polyelectrolyte Assemblies: Effective Photocatalysts in Solution. Polymers. 2016; 8(5):180. https://doi.org/10.3390/polym8050180
Chicago/Turabian StyleFrühbeißer, Sabine, Giacomo Mariani, and Franziska Gröhn. 2016. "Porphyrin Diacid-Polyelectrolyte Assemblies: Effective Photocatalysts in Solution" Polymers 8, no. 5: 180. https://doi.org/10.3390/polym8050180
APA StyleFrühbeißer, S., Mariani, G., & Gröhn, F. (2016). Porphyrin Diacid-Polyelectrolyte Assemblies: Effective Photocatalysts in Solution. Polymers, 8(5), 180. https://doi.org/10.3390/polym8050180