Hydrogel is Superior to Fibrin Gel as Matrix of Stem Cells in Alleviating Antigen-Induced Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
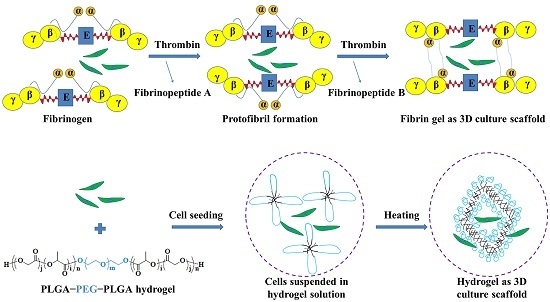
2.2. Preparation of Fibrin Gel
2.3. Preparation of PLGA−PEG−PLGA Hydrogel
2.4. Morphology and Dynamic Mechanical Analyses
2.5. Isolation and Culture of BMMSCs
2.6. Cell Encapsulation and DAPI Staining
2.7. OVA-Induced Arthritis in Rabbits
2.8. Surgical Procedure and Cell Transplantation
2.9. Measurement of Joint Swelling
2.10. Detection of Cytokines in Serum
2.11. Gross Morphologies
2.12. Histological and Immunohistochemical Analyses
2.13. Statistical Analyses
3. Results and Discussion
3.1. Morphology and Cell Distribution of Scaffolds
3.2. Rheological Analyses
3.3. Clinical Manifestations Regulated by Cell Transplantation
3.4. Level Alterations of Serum Inflammatory Cytokines
3.5. Amelioration of Arthritis Mediated by Different Scaffolds-Assisted BMMSCs Transplantation
3.5.1. Efficacy of Cartilage Repair in Created Defects
3.5.2. Protection of Tissues Surrounding Cartilage
3.5.3. Inhibition of Synovial Hyperplasia and Inflammatory Performance
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pap, T.; Korb-Pap, A. Cartilage damage in osteoarthritis and rheumatoid arthritis—Two unequal siblings. Nat. Rev. Rheumatol. 2015, 11, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Cancedda, R.; Bianchi, G.; Derubeis, A.; Quarto, R. Cell therapy for bone disease: A review of current status. Stem Cells 2003, 21, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Prockop, D.J. Concise review: Mesenchymal stem/multipotent stromal cells: The state of transdifferentiation and modes of tissue repair-current views. Stem Cells 2007, 25, 2896–2902. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, M.P.; Fuentes-Julián, S.; Blázquez-Martínez, A.; Pascual, C.Y.; Aller, M.A.; Arias, J.; Arnalich-Montiel, F. Immunosuppressive properties of mesenchymal stem cells: Advances and applications. Curr. Mol. Med. 2012, 12, 574–591. [Google Scholar] [PubMed]
- Abumaree, M.; Al Jumah, M.; Pace, R.A.; Kalionis, B. Immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev. 2012, 8, 375–392. [Google Scholar] [CrossRef] [PubMed]
- Augello, A.; Tasso, R.; Negrini, S.M.; Cancedda, R.; Pennesi, G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007, 56, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.A.; Gonzalez-Rey, E.; Rico, L.; Buscher, D.; Delgado, M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009, 60, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Xu, W.R.; Qian, H.; Zhu, W.; Yan, Y.M.; Shao, Q.X.; Xu, H.X. Immunosuppressive effects of mesenchymal stem cells in collagen-induced mouse arthritis. Inflamm. Res. 2010, 59, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Keerthi, N.; Chimutengwende-Gordon, M.; Sanghani, A.; Khan, W. The potential of stem cell therapy for osteoarthritis and rheumatoid arthritis. Curr. Stem Cell Res. Ther. 2013, 8, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, N. Cartilage degradation in rheumatoid arthritis. Clin. Calcium. 2009, 19, 347–354. [Google Scholar] [PubMed]
- Liu, H.; Ding, J.; Wang, J.; Wang, Y.; Yang, M.; Zhang, Y.; Chang, F.; Chen, X. Remission of collagen-induced arthritis through combination therapy of microfracture and transplantation of thermogel-encapsulated bone marrow mesenchymal stem cells. PloS ONE 2015, 10, e0120596. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ding, J.; Wang, C.; Wang, J.; Wang, Y.; Yang, M.; Jia, Y.; Zhang, Y.; Chang, F.; Li, R.; et al. Intra-articular transplantation of allogeneic BMMSCs rehabilitates cartilage injury of antigen-induced arthritis. Tissue Eng. Part A 2015, 21, 2733–2743. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Shi, K.; Ding, Q.; Qu, Y.; Luo, F.; Qian, Z. Recent developments in scaffold-guided cartilage tissue regeneration. J. Biomed. Nanotechnol. 2014, 10, 3085–3104. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.J.; Mahapatra, C.; Singh, R.K.; Knowles, J.C.; Kim, H.W. Strategies for osteochondral repair: Focus on scaffolds. J. Tissue Eng. 2014, 5, 2041731414541850. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, S.N. Cell electrospinning: A novel tool for functionalising fibres, scaffolds and membranes with living cells and other advanced materials for regenerative biology and medicine. Analyst 2013, 138, 2215–2223. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meng, H.; Liu, Y.; Lee, B.P. Fibrin gel as an injectable biodegradable scaffold and cell carrier for tissue engineering. Sci. World J. 2015, 2015, 685690. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Maher, S.A.; Lowman, A.M. Hydrogels for the repair of articular cartilage defects. Tissue Eng. B 2011, 17, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Peng, S.; Chen, A.; Li, F.; Ren, K.; Hu, N. Biocompatibility studies on fibrin glue cultured with bone marrow mesenchymal stem cells in vitro. J Huazhong Univ. Sci. Technol. Med. Sci. 2004, 24, 272–274. [Google Scholar] [PubMed]
- Silverman, R.P.; Passaretti, D.; Huang, W.; Randolph, M.A.; Yaremchuk, M.J. Injectable tissue-engineered cartilage using a fibrin glue polymer. Plast Reconstr. Surg. 1999, 103, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Ishii, T.; Yanai, T.; Mishima, H.; Akaogi, H.; Ogawa, T.; Ochiai, N. Repair of large full-thickness articular cartilage defects by transplantation of autologous uncultured bone-marrow-derived mononuclear cells. J. Orthop. Res. 2008, 26, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ding, J.; Zhang, Z.; Yang, M.; Yu, J.; Wang, J.; Chang, F.; Chen, X. Kartogenin-incorporated thermogel supports stem cells for significant cartilage regeneration. ACS Appl. Mater. Interfaces 2016, 8, 5148–5159. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Kwon, I.K.; Park, K. Hydrogels for delivery of bioactive agents: A historical perspective. Adv. Drug Deliv. Rev. 2013, 65, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Ding, J.X.; Xu, W.G.; Wu, J.; Chang, F.; Zhuang, X.L.; Chen, X.S.; Wang, J.C. Biodegradable thermogel as culture matrix of bone marrow mesenchymal stem cells for potential cartilage tissue engineering. Chin. J. Polym. Sci. 2014, 32, 1590–1601. [Google Scholar] [CrossRef]
- Van den Borne, M.P.; Raijmakers, N.J.; Vanlauwe, J.; Victor, J.; de Jong, S.N.; Bellemans, J.; Saris, D.B.; International Cartilage Repair Society. International Cartilage Repair Society (ICRS) and Oswestry macroscopic cartilage evaluation scores validated for use in Autologous Chondrocyte Implantation (ACI) and microfracture. Osteoarthritis Cartilage 2007, 15, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.; Yan, S.; Gong, L.; Wang, J.; Chen, X.; Cui, L.; Yin, J. Repair of an articular cartilage defect using adipose-derived stem cells loaded on a polyelectrolyte complex scaffold based on poly(l-glutamic acid) and chitosan. Acta Biomater. 2013, 9, 7276–7288. [Google Scholar] [CrossRef] [PubMed]
- Cake, M.A.; Read, R.A.; Guillou, B.; Ghosh, P. Modification of articular cartilage and subchondral bone pathology in an ovine meniscectomy model of osteoarthritis by avocado and soya unsaponifiables (ASU). Osteoarthritis Cartilage 2000, 8, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Krenn, V.; Morawietz, L.; Haupl, T.; Neidel, J.; Petersen, I.; König, A. Grading of chronic synovitis—A histopathological grading system for molecular and diagnostic pathology. Pathol. Res. Pract. 2002, 198, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Feng, G.; Yan, W. Mesenchymal stem cell-based treatment for cartilage defects in osteoarthritis. Mol. Biol. Rep. 2012, 39, 5683–5689. [Google Scholar] [CrossRef] [PubMed]
- Nukavarapu, S.P.; Dorcemus, D.L. Osteochondral tissue engineering: Current strategies and challenges. Biotechnol. Adv. 2013, 31, 706–721. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, R.J.; Graham, S.M.; Davies, P.S.; Korres, N.; Tsouchnica, H.; Heliotis, M.; Mantalaris, A.; Tsiridis, E. Anti-inflammatory role and immunomodulation of mesenchymal stem cells in systemic joint diseases: Potential for treatment. Expert Opin. Ther. Targets 2013, 17, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, O.; Cartwright, A.; Askari, A.; El Haj, A.J.; Middleton, J. Intra-articular injection of mesenchymal stem cells leads to reduced inflammation and cartilage damage in murine antigen-induced arthritis. J. Transl. Med. 2014, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, O.; Babakurban, S.T.; Akkuzu, H.G.; Bilgi, S.; Ovalı, E.; Kongur, M.; Altintas, H.; Yilmaz, B.; Bilezikçi, B.; Celik, Z.Y.; Yakicier, M.C.; Sahin, F.I. Injectable tissue-engineered cartilage using commercially available fibrin glue. Laryngoscope 2013, 123, 2986–2992. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.A. The role of T cells in the immunopathogenesis of rheumatoid arthritis: New perspectives. Arthritis Rheum. 1997, 40, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Abramson, S.B.; Pillinger, M.H. The fibroblast-like synovial cell in rheumatoid arthritis: A key player in inflammation and joint destruction. Clin. Immunol. 2005, 115, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Eren, G.; Gurkan, A.; Atmaca, H.; Donmez, A.; Atilla, G. Effect of centrifugation time on growth factor and MMP release of an experimental platelet-rich fibrin-type product. Platelets 2016, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Thornton, S.C.; Por, S.B.; Penny, R.; Richter, M.; Shelley, L.; Breit, S.N. Identification of the major fibroblast growth factors released spontaneously in inflammatory arthritis as platelet derived growth factor and tumour necrosis factor-alpha. Clin. Exp. Immunol. 1991, 86, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Remmers, E.F.; Sano, H.; Wilder, R.L. Platelet-derived growth factors and heparin-binding (fibroblast) growth factors in the synovial tissue pathology of rheumatoid arthritis. Semin. Arthritis Rheum. 1991, 21, 191–199. [Google Scholar] [CrossRef]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.K.; Flavell, R.A. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.M.; Allen, J.B.; Wong, H.L.; Dougherty, S.F.; Ellingsworth, L.R. Antagonistic and agonistic effects of transforming growth factor-β and IL-1 in rheumatoid synovium. J. Immunol. 1990, 145, 2514–2519. [Google Scholar]
- Yamanishi, Y.; Boyle, D.L.; Clark, M.; Maki, R.A.; Tortorella, M.D.; Arner, E.C.; Firestein, G.S. Expression and regulation of aggrecanase in arthritis: The role of TGF-β. J. Immunol. 2002, 168, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Thorbecke, G.J.; Shah, R.; Leu, C.H.; Kuruvilla, A.P.; Hardison, A.M.; Palladino, M.A. Involvement of endogenous tumor necrosis factor alpha and transforming growth factor beta during induction of collagen type II arthritis in mice. Proc. Natl. Acad. Sci. USA 1992, 15, 89–7375. [Google Scholar] [CrossRef]
- Allen, J.B.; Manthey, C.L.; Hand, A.R.; Ohura, K.; Ellingsworth, L.; Wahl, S.M. Rapid onset synovial inflammation and hyperplasia induced by transforming growth factor beta. J. Exp. Med. 1990, 171, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, S.; Corr, M.; Boyle, D.L. Platelet-derived growth factor and transforming growth factor β synergistically potentiate inflammatory mediator synthesis by fibroblast-like synoviocytes. Arthritis Res. Ther. 2010, 12, R65. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, E.; Canete, J.D.; Celis, R.; Del Rey, M.J.; Usategui, A.; Marsal, S.; Sanmartí, R.; Criado, G.; Pablos, J.L. Synovial fibroblast hyperplasia in rheumatoid arthritis: Clinicopathologic correlations and partial reversal by anti-tumor necrosis factor therapy. Arthritis Rheum. 2011, 63, 2575–2583. [Google Scholar] [CrossRef] [PubMed]
- Del Rey, M.J.; Izquierdo, E.; Caja, S.; Usategui, A.; Santiago, B.; Galindo, M.; Pablos, J.L. Human inflammatory synovial fibroblasts induce enhanced myeloid cell recruitment and angiogenesis through a hypoxia-inducible transcription factor 1alpha/vascular endothelial growth factor-mediated pathway in immunodeficient mice. Arthritis Rheum. 2009, 60, 2926–2934. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.D.; Filer, A.; Haworth, O.; Parsonage, G.; Salmon, M. Defining a role for fibroblasts in the persistence of chronic inflammatory joint disease. Ann. Rheum. Dis. 2004, 63, ii92–ii95. [Google Scholar] [CrossRef] [PubMed]










© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Ding, J.; Li, C.; Wang, C.; Wang, Y.; Wang, J.; Chang, F. Hydrogel is Superior to Fibrin Gel as Matrix of Stem Cells in Alleviating Antigen-Induced Arthritis. Polymers 2016, 8, 182. https://doi.org/10.3390/polym8050182
Liu H, Ding J, Li C, Wang C, Wang Y, Wang J, Chang F. Hydrogel is Superior to Fibrin Gel as Matrix of Stem Cells in Alleviating Antigen-Induced Arthritis. Polymers. 2016; 8(5):182. https://doi.org/10.3390/polym8050182
Chicago/Turabian StyleLiu, He, Jianxun Ding, Chen Li, Chenyu Wang, Yinan Wang, Jincheng Wang, and Fei Chang. 2016. "Hydrogel is Superior to Fibrin Gel as Matrix of Stem Cells in Alleviating Antigen-Induced Arthritis" Polymers 8, no. 5: 182. https://doi.org/10.3390/polym8050182
APA StyleLiu, H., Ding, J., Li, C., Wang, C., Wang, Y., Wang, J., & Chang, F. (2016). Hydrogel is Superior to Fibrin Gel as Matrix of Stem Cells in Alleviating Antigen-Induced Arthritis. Polymers, 8(5), 182. https://doi.org/10.3390/polym8050182